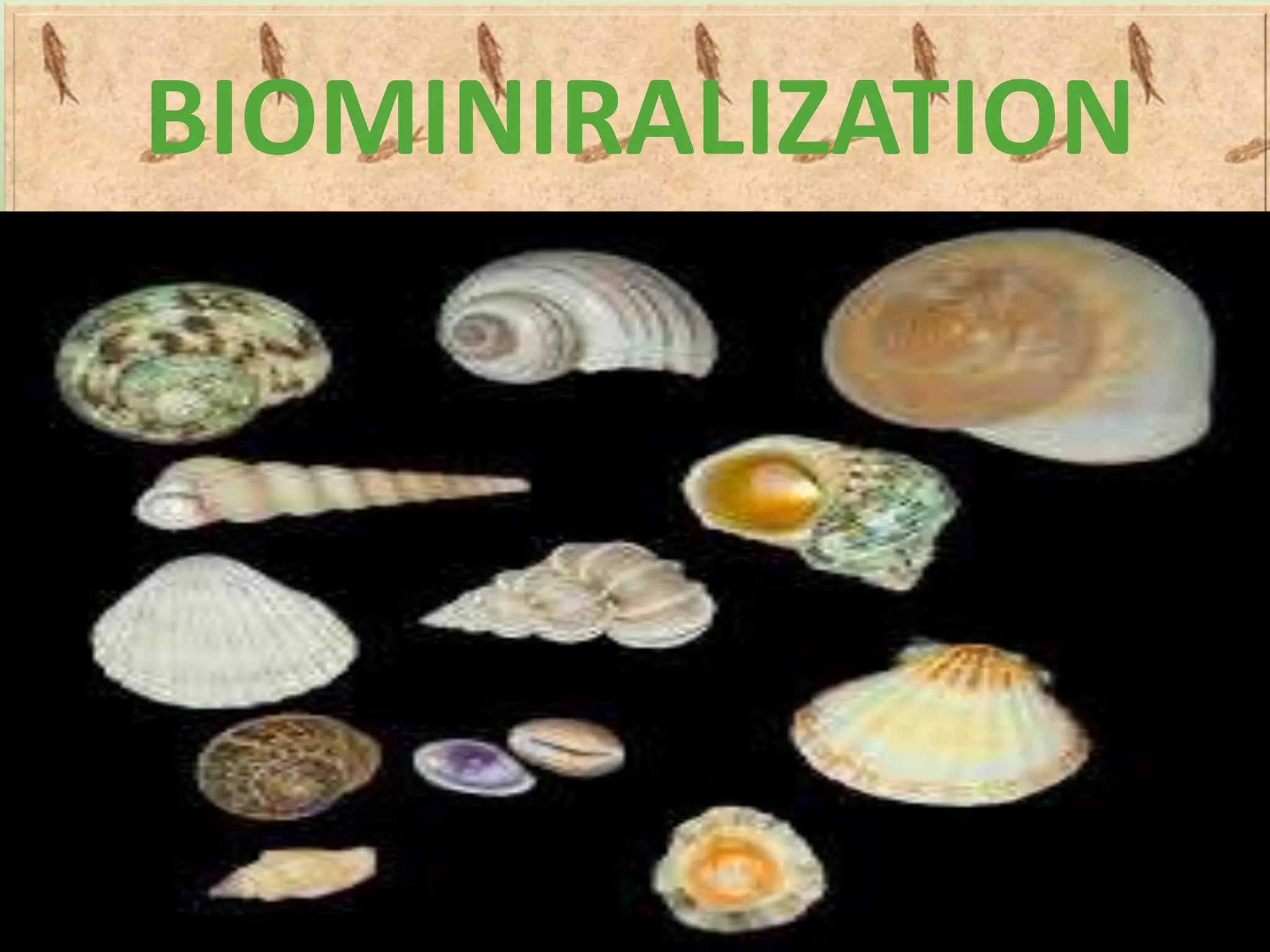
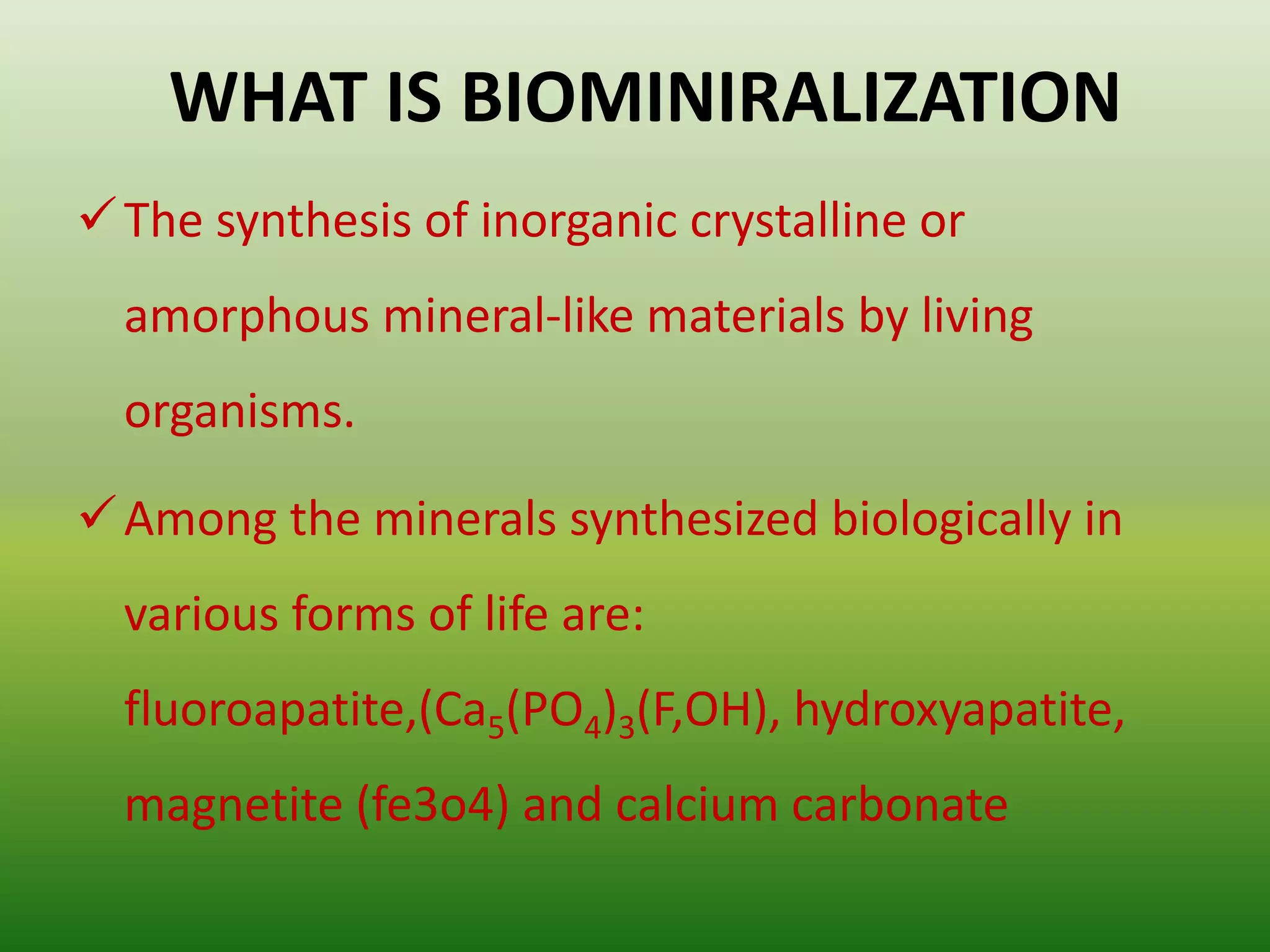
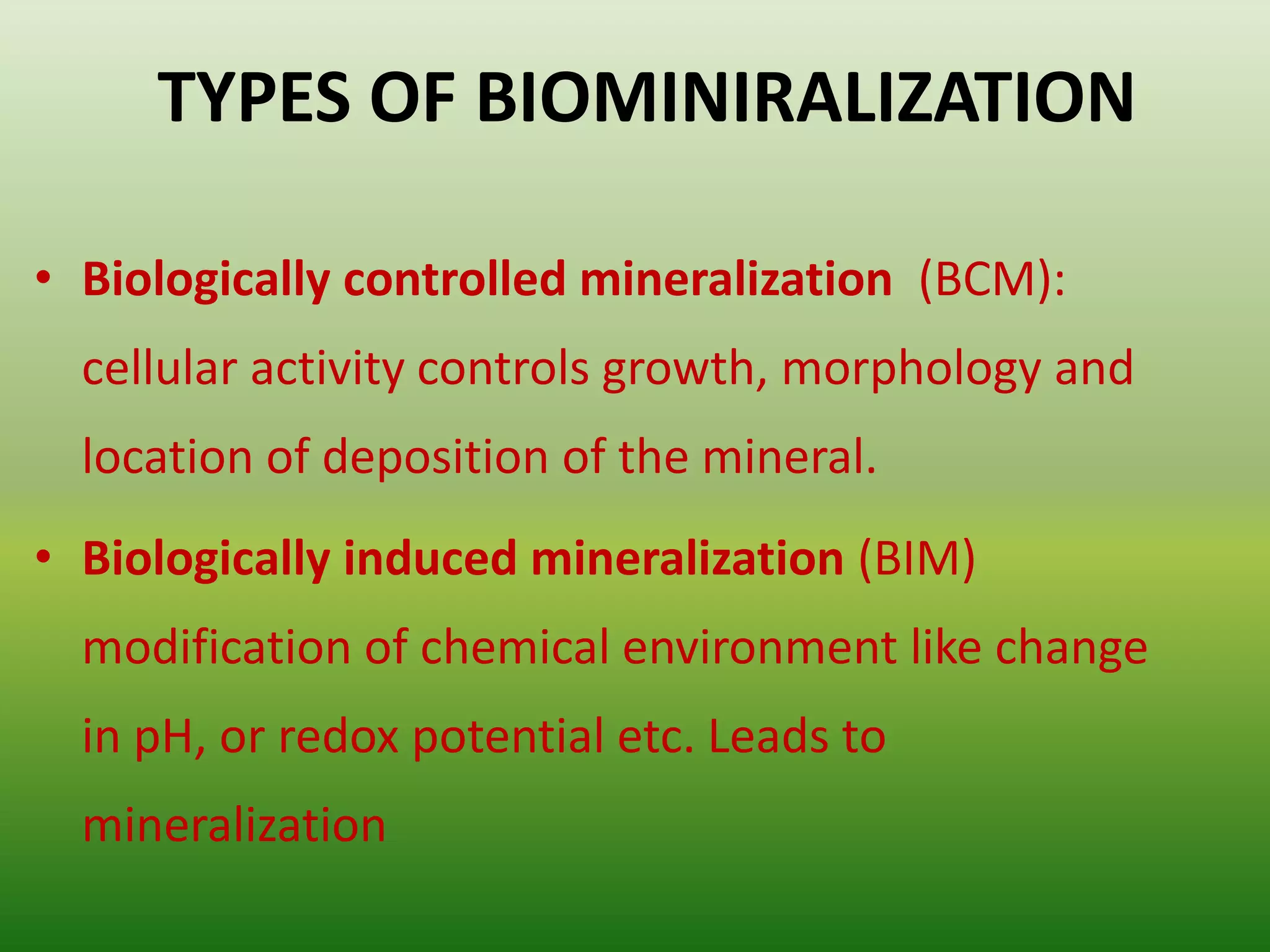
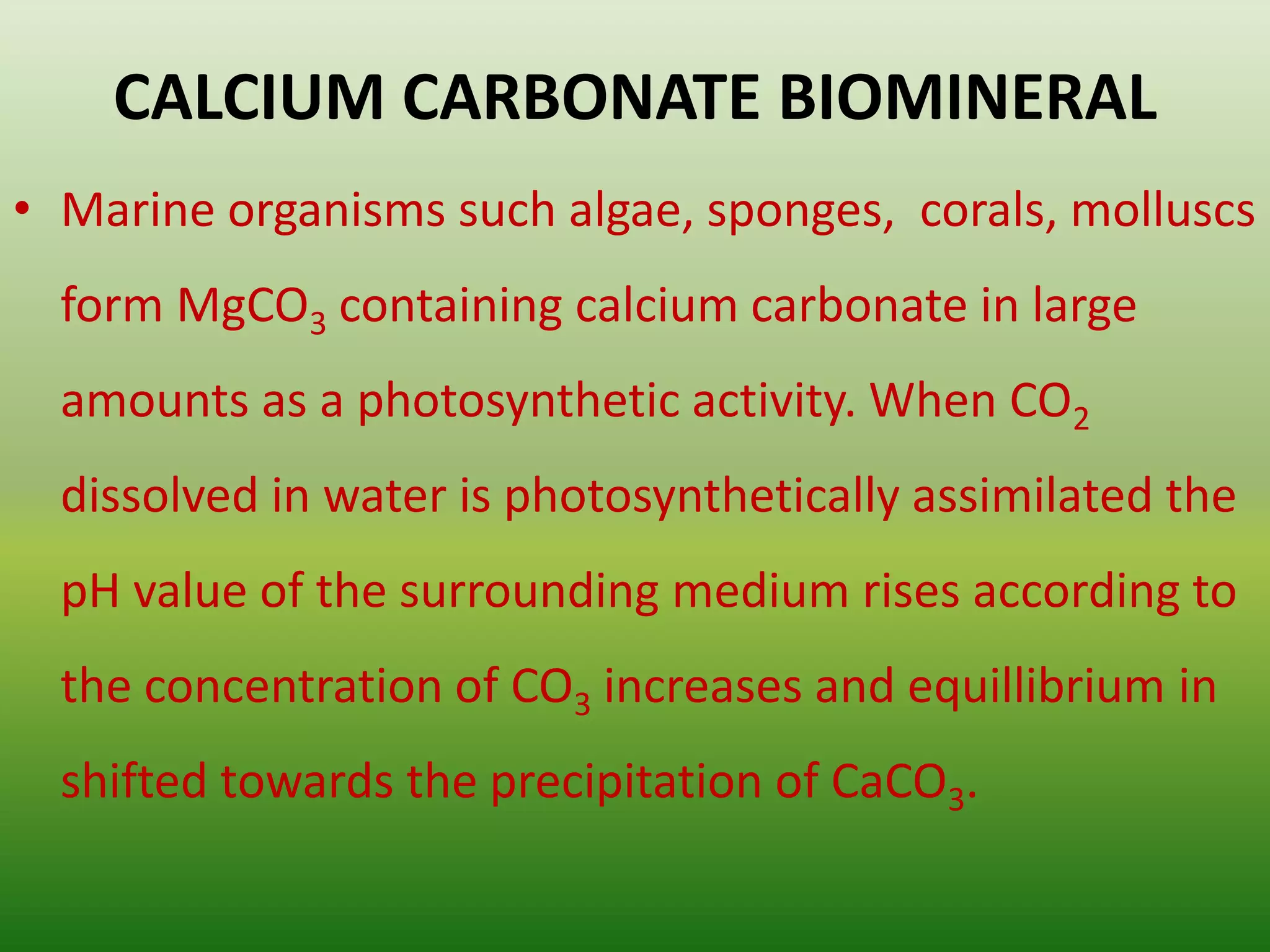
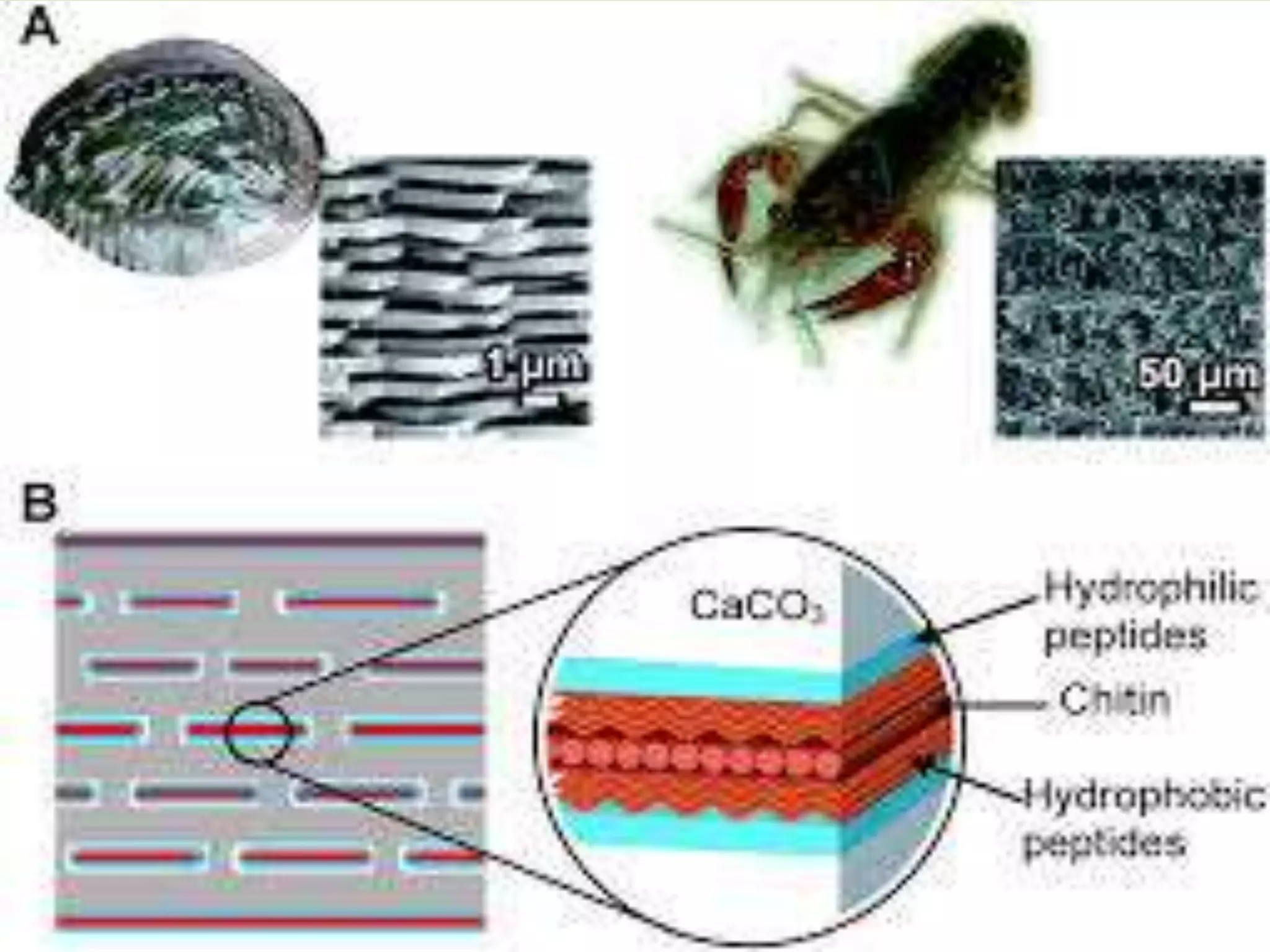
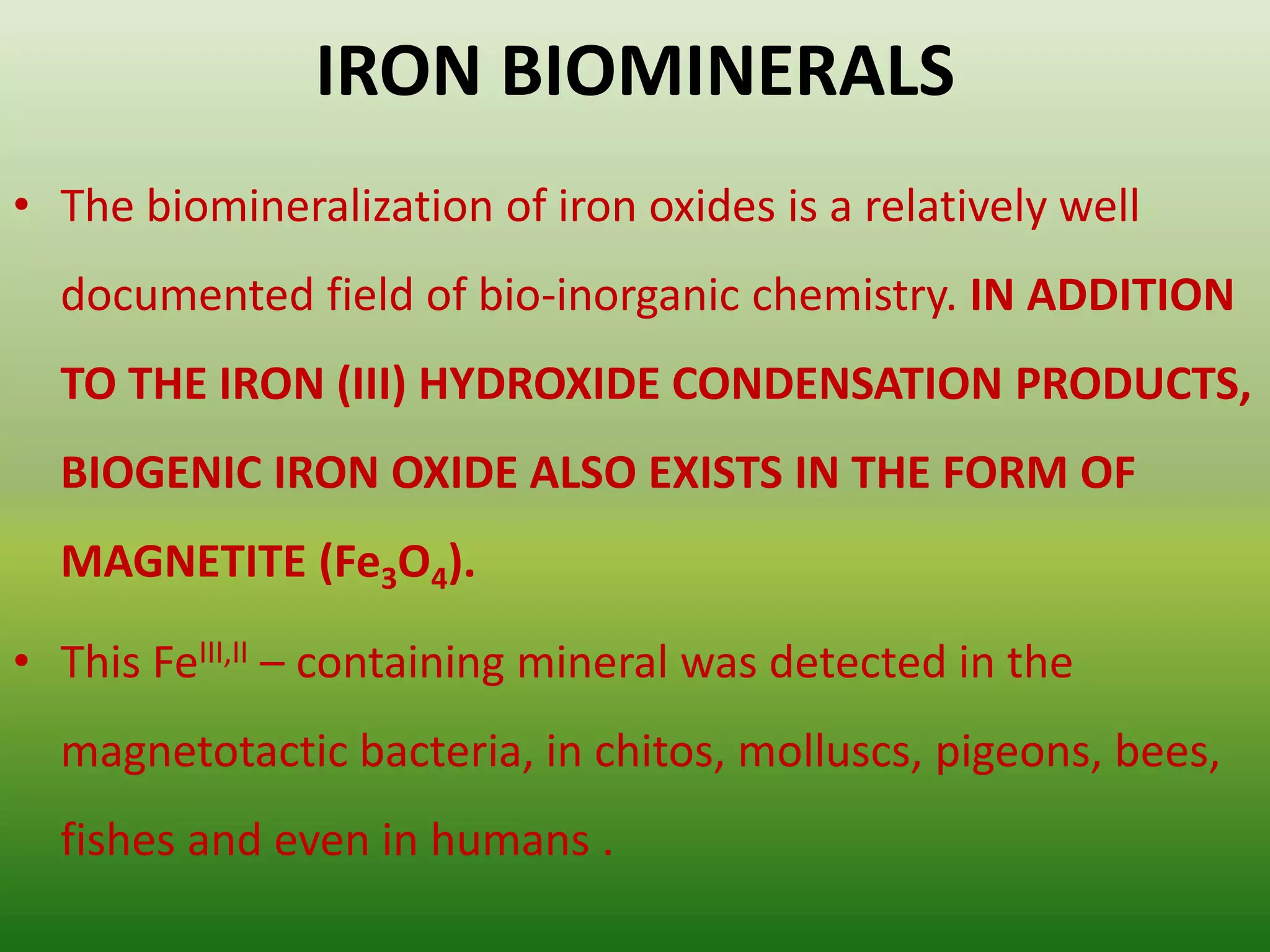
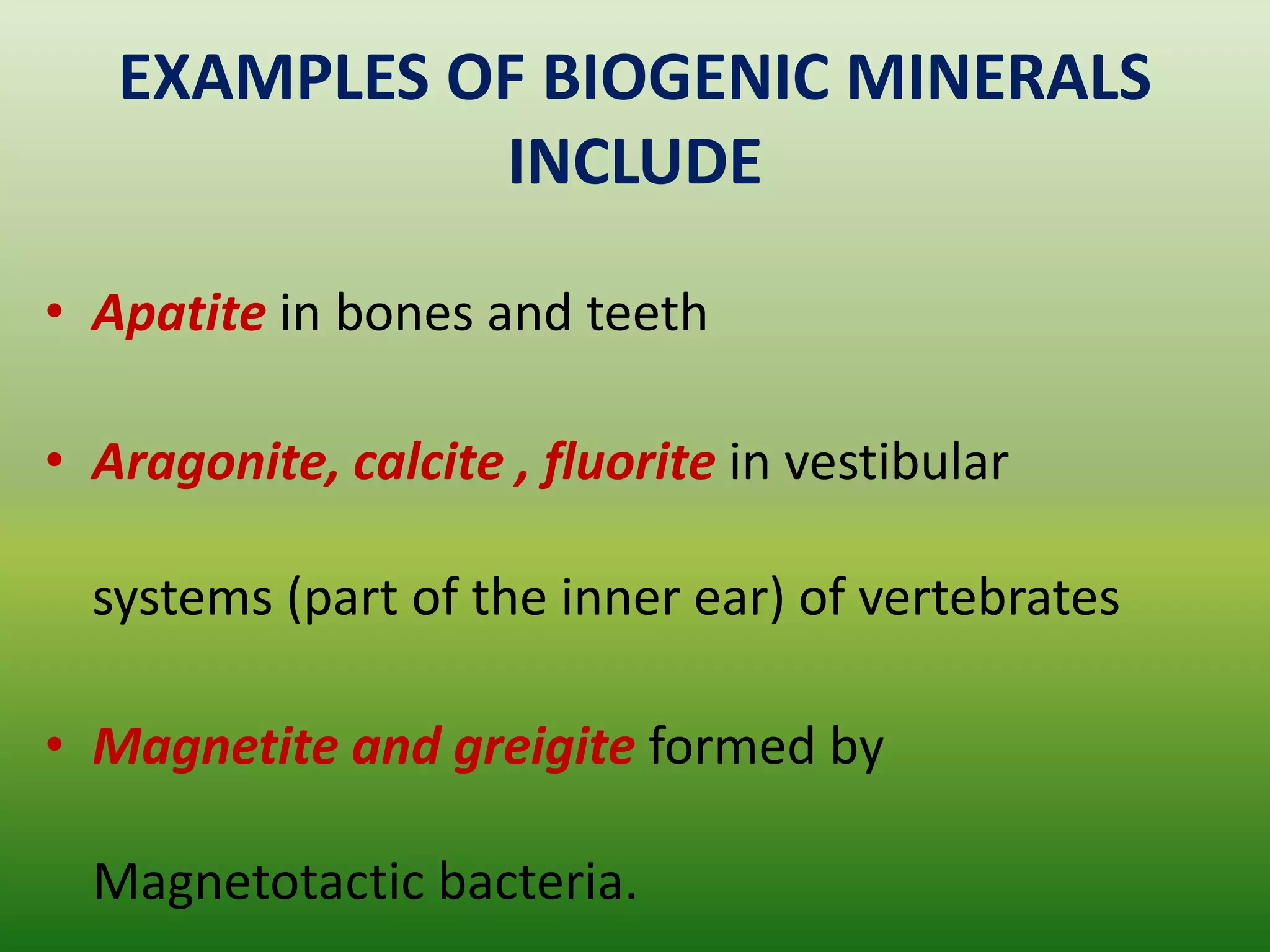
Biomineralization is the process by which living organisms produce minerals. It occurs through two main types: biologically controlled mineralization, where the organism controls mineral growth and placement, and biologically induced mineralization, where the organism modifies the environment to induce mineral formation. Calcium carbonate is a common biomineral found in marine organisms through photosynthesis raising the pH and carbonate concentration to precipitate calcium carbonate. Magnetite is also an important biomineral formed by magnetotactic bacteria, which synthesize chains of magnetic crystals called magnetosomes that act as a biological compass. Examples of other biogenic minerals include apatite in bones/teeth and iron oxides formed by various organisms.