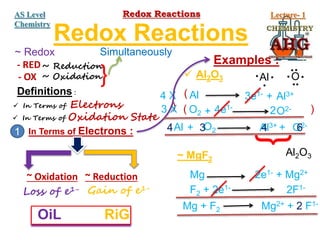
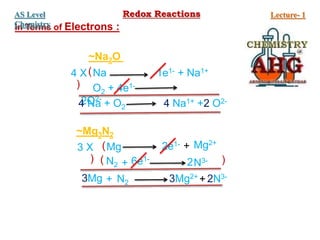
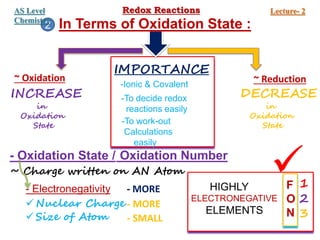
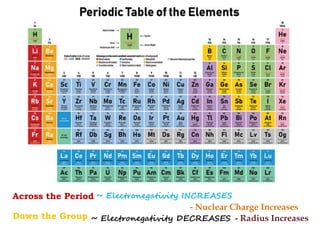
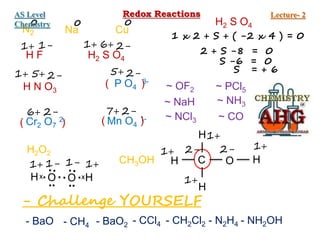
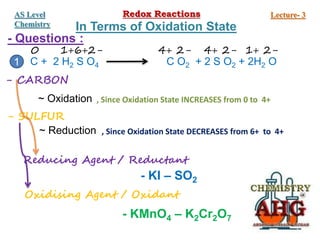
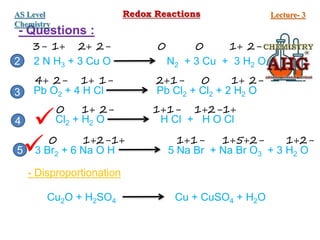
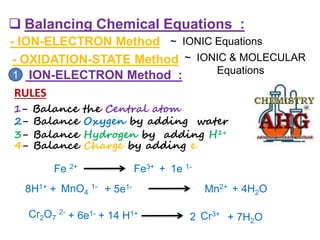
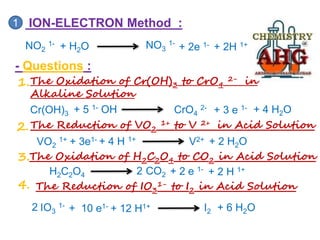
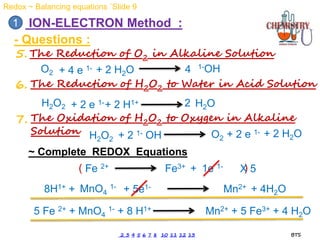
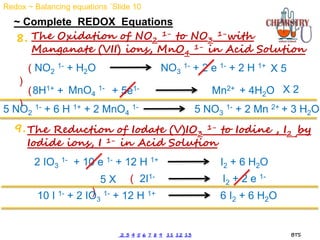
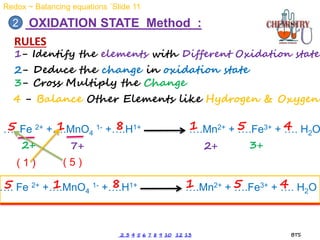
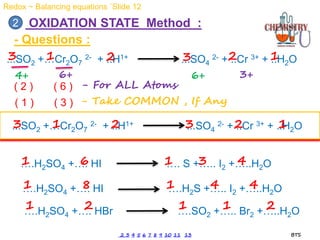
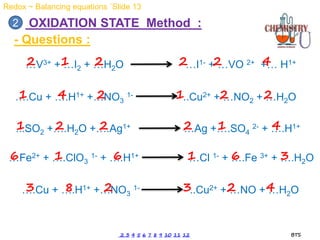
Redox reactions involve the simultaneous oxidation and reduction of reactants. Oxidation is defined as an increase in oxidation state or loss of electrons, while reduction is a decrease in oxidation state or gain of electrons. There are two main methods for balancing redox reactions - the ion-electron method which balances the ions and electrons, and the oxidation state method which balances the changes in oxidation states of elements between reactants and products. Balancing redox reactions involves identifying changes in oxidation states, cross multiplying these changes, and then balancing other elements such as hydrogen and oxygen.