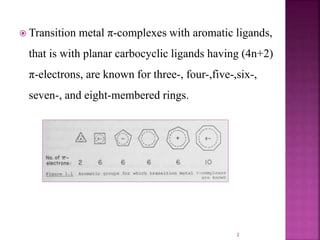
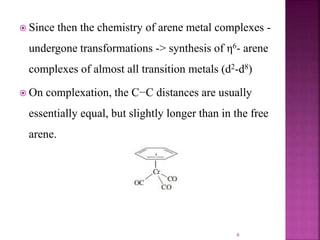
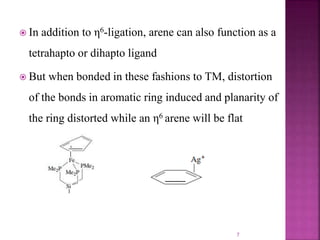
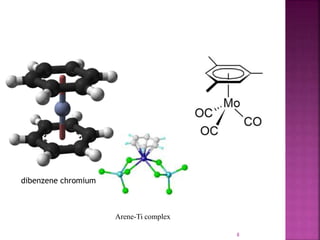
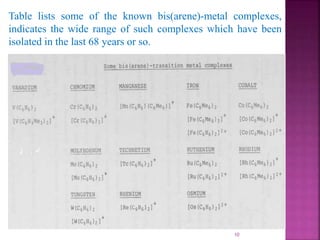
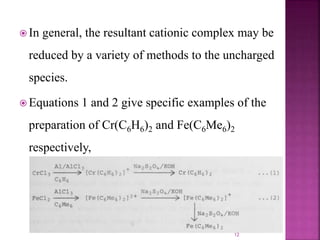
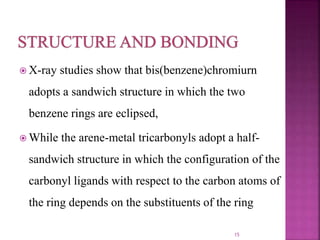
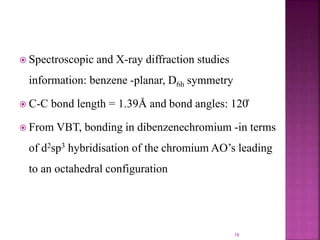
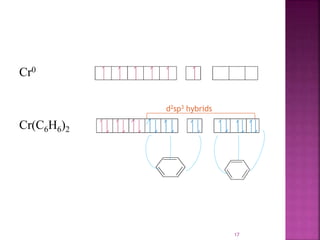
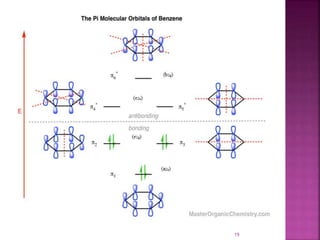
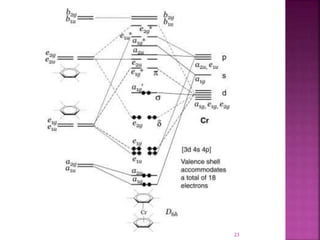
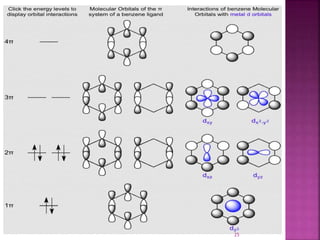
The document summarizes transition metal complexes containing aromatic ligands. It discusses how transition metals form pi complexes with planar carbocyclic ligands having (4n+2) pi electrons. Cyclopentadienyl complexes are known for most transition metals. The number of stable complexes decreases in the order C5H5->C6H6->C7H7+->C9H9. Arenes can bind in eta6, tetrahapto, or dihapto modes. Characterization is often done using 13C NMR which shows a shift for metal-bound carbons.








![ More reactive than metallocenes, thermally and
aerially less stable
Cr(η6-C6H6)2 readily oxidises to [Cr(η6-C6H6)2]+
Cr(C6H6)2 [Cr(C6H6)2]+
+ C2H5
.
C2H5I
(C2H6 + C2H4 + C4H10)
Disproportionation/addition
27](https://image.slidesharecdn.com/arene-metalcomplex-240412112913-d8a44e13/85/Powerpoint-on-Arene-Metal-complexes-pptx-27-320.jpg)