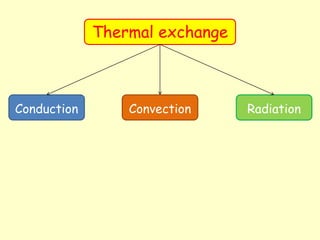
This document discusses different methods of thermal energy transfer: conduction, convection, and radiation.
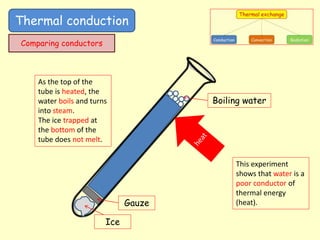
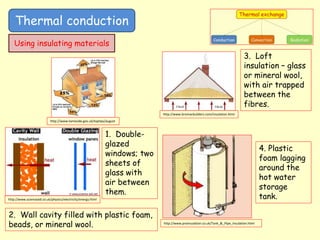
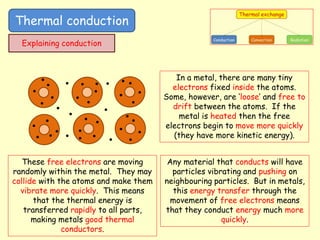
[1] Thermal conduction involves the transfer of kinetic energy between particles in direct contact. Metals are good conductors as their free electrons can rapidly transfer energy. Insulators have trapped pockets of air that slow conduction.
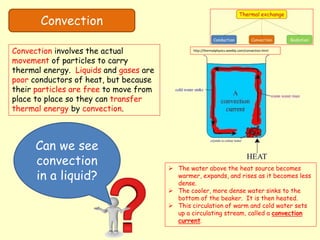
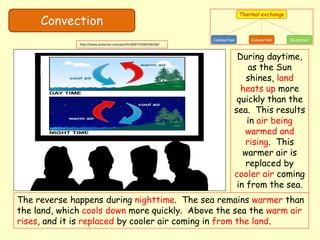
[2] Convection relies on the actual movement of heated liquid or gas particles. Circulation patterns in liquids and winds in the atmosphere transfer thermal energy over distances.
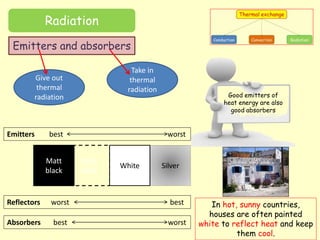
[3] Radiation involves the emission and absorption of electromagnetic waves and does not require a medium for transfer. All objects radiate energy based on their temperature, with better emitters also being good absorbers.