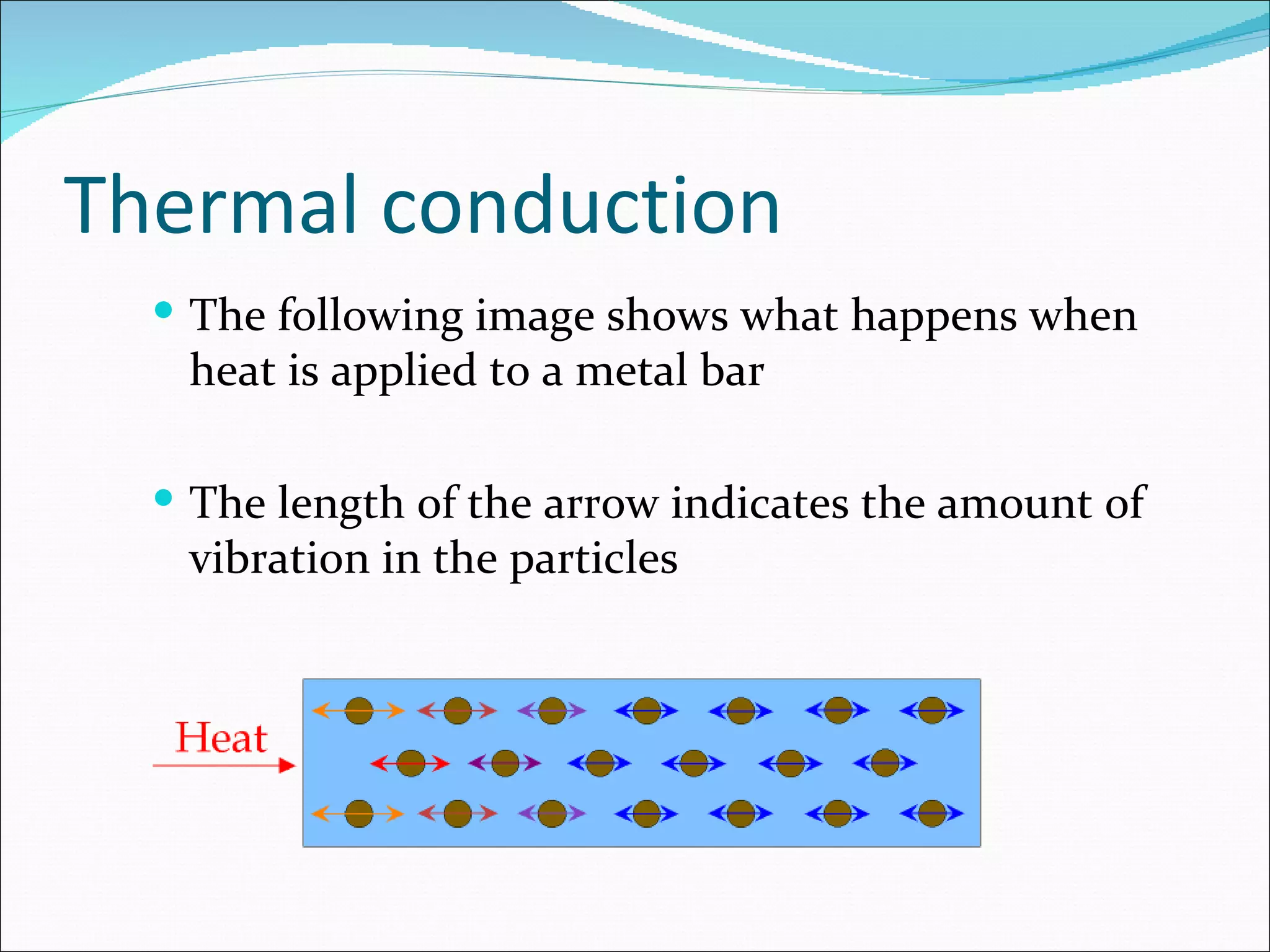
Thermal energy can be transferred between substances in three ways: conduction, convection, and radiation. Conduction involves the transfer of kinetic energy between particles in solids and liquids when one end is heated. The rate of heat transfer by conduction depends on the temperature difference and the material's specific heat capacity, which is the amount of energy needed to change its temperature. Heat always flows naturally from hotter to cooler substances until thermal equilibrium is reached.