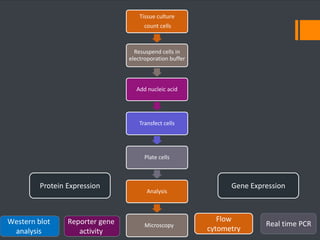
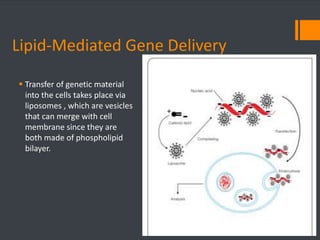
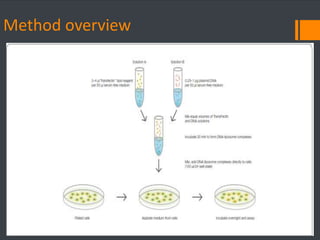
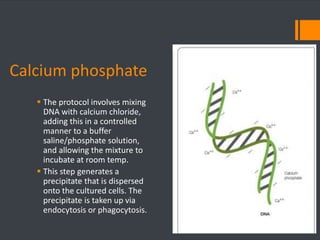
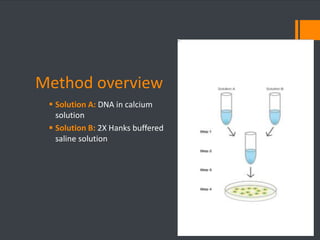
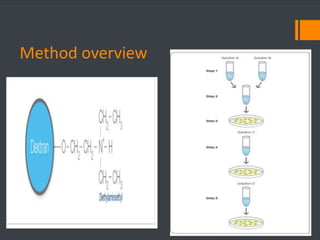
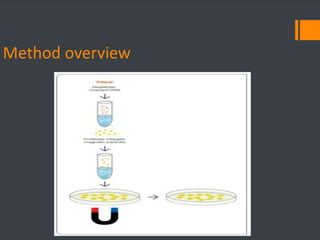
This document discusses various chemical agents and methods used for cell transformation through transfection of foreign DNA. It describes factors that affect transfection efficiency, including host cell health and culture conditions as well as DNA quality and quantity. Common transfection methods are also outlined, such as cationic lipids, calcium phosphate, DEAE-Dextran, and magnet-mediated transfection. Each method is briefly explained and its pros and cons discussed.