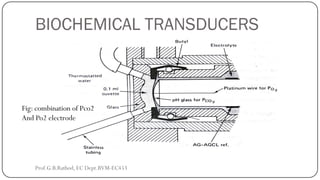
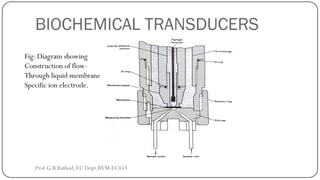
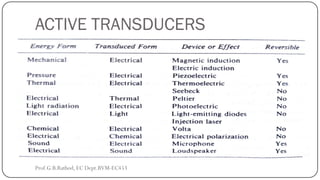
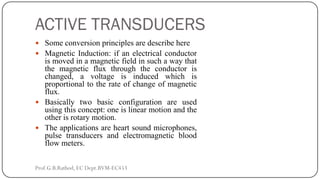
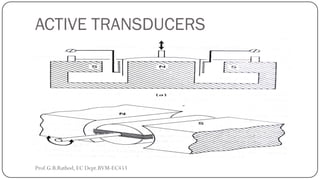
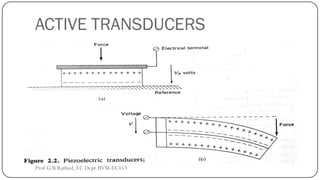
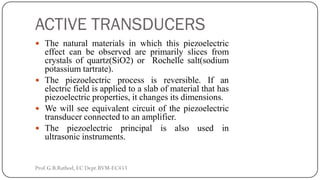
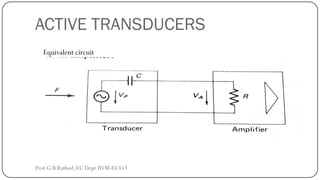
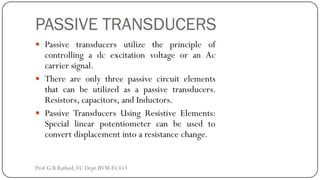
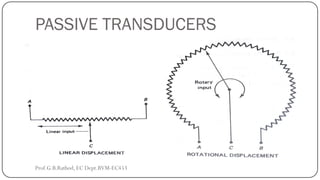
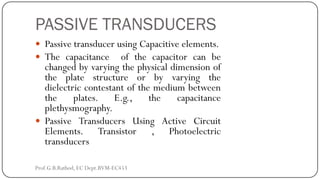
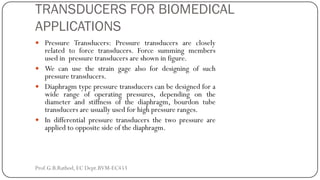
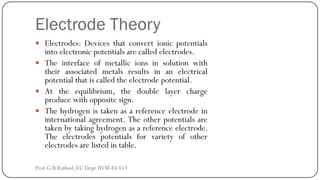
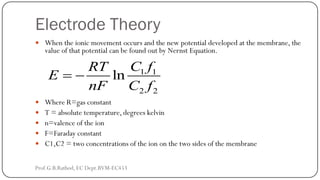
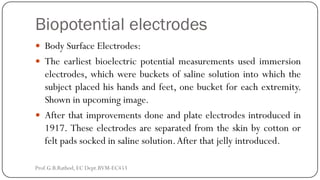
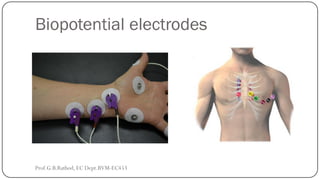
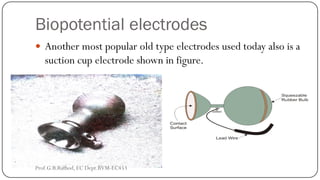
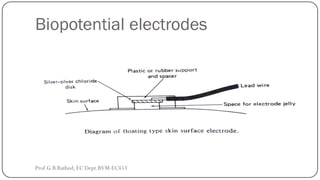
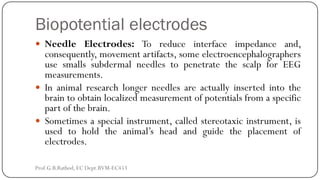
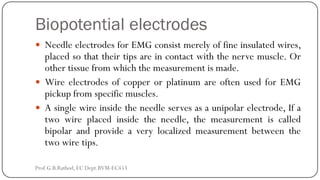
This document discusses basic transducer principles and biopotential electrodes. It covers the definitions of a transducer and transduction as the conversion of one form of energy to another. Two main principles of transduction are discussed: active transducers using energy conversion and passive transducers using modulation. Examples of active transducers include those using magnetic induction, piezoelectricity, and thermoelectric effects. Passive transducers can use resistive, capacitive, or inductive elements. The document also covers principles of biopotential electrodes including types for measuring signals on the skin surface, within tissues using needles, and within single cells using microelectrodes. Equivalent circuits and considerations for reducing noise



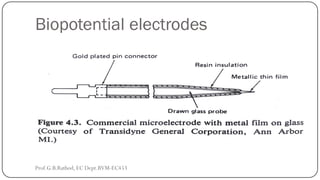

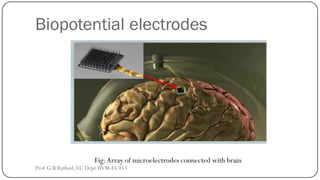
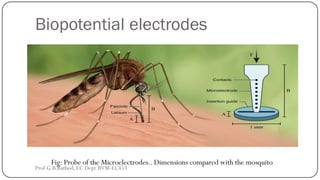
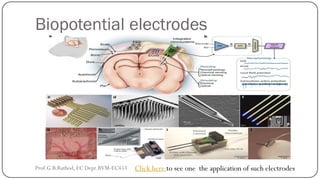












![BIOCHEMICAL TRANSDUCERS
The pH Electrode
To know chemical balance in the body, pH of the blood and other fluids
are very important.
Equation of pH is
pH is a measure of the acid base balance of a fluid.
A natural solution has a pH of 7. Lower pH numbers indicate acidity,
whereas higher pH values define a basic solution. Most human body
fluids are slightly basic. The pH of normal arterial blood ranges between
7.38 and 7.42. The pH of venous blood is 7.35, because of the extra
CO2.
10 10
1
log [ ] log
[ ]
pH H
H
Prof.G.B.Rathod, EC Dept.BVM-EC453](https://image.slidesharecdn.com/topic3basictransducerprinciples-210210061130/85/Topic-3-Basic-transducer-principles-Electrodes-53-320.jpg)