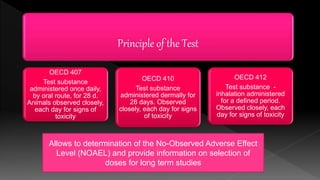
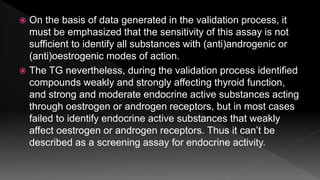
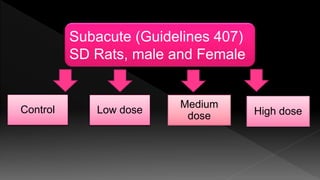
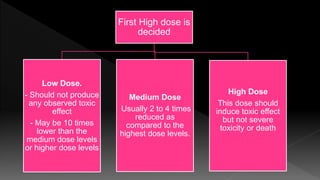
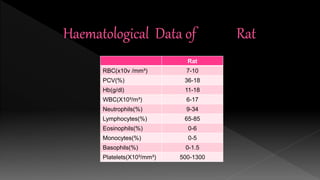
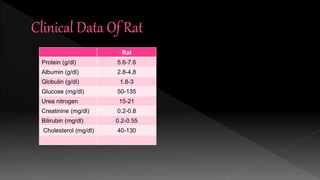
The document outlines the principles and protocols for toxicology testing, particularly the OECD test guidelines for a 28-day repeated dose toxicity study in rodents, focusing on safety assessment for potential drugs. It discusses various toxicity testing types, animal care conditions, dosages, and monitoring practices to identify adverse effects, including neurotoxic and endocrine disruptor evaluations. Key parameters for data collection and analysis for hazard identification and risk assessment are also summarized.