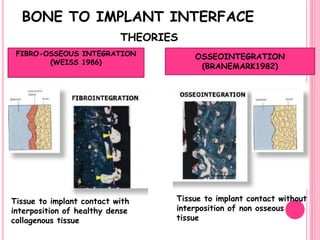
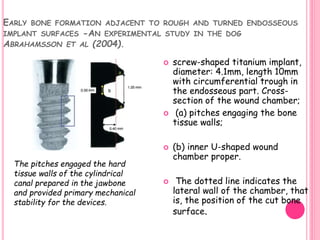
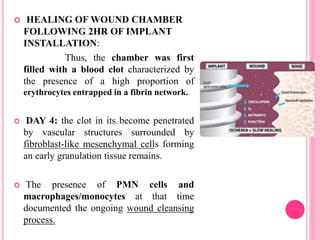
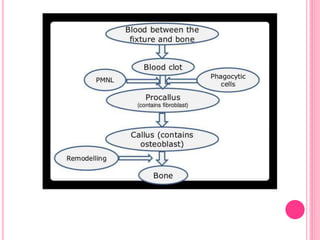
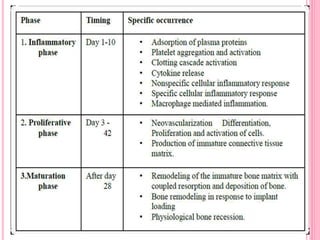
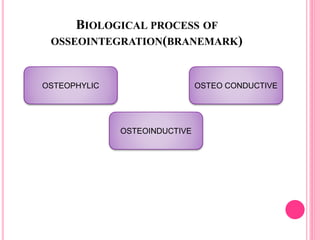
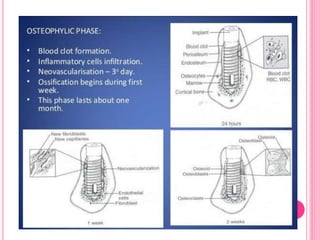
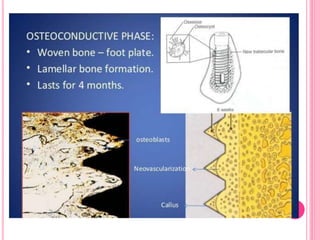
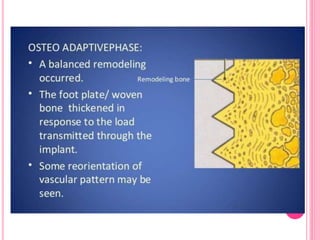
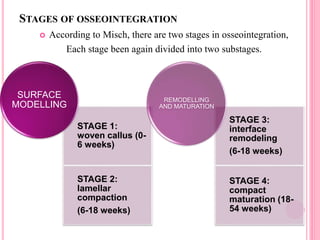
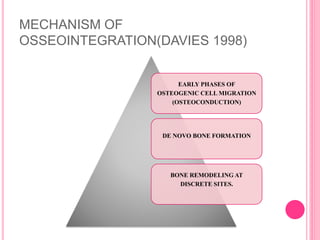
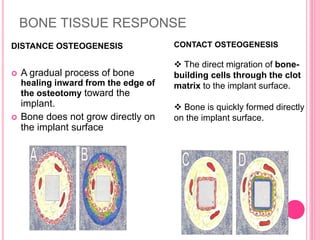
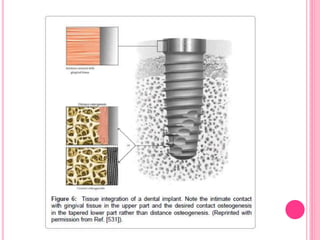
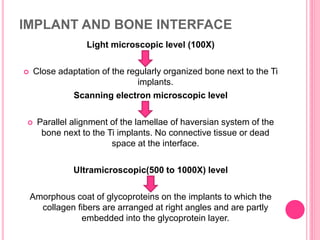
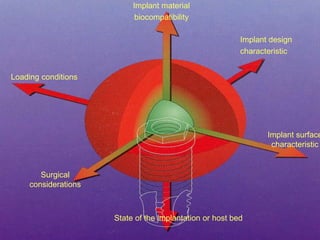
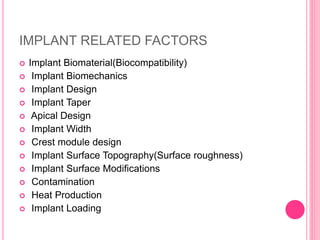
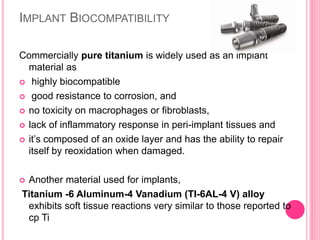
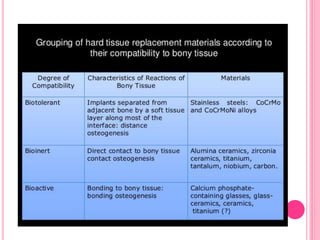
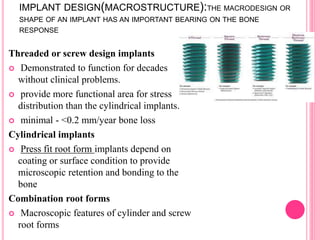
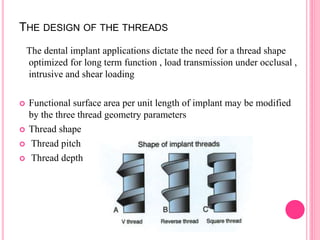
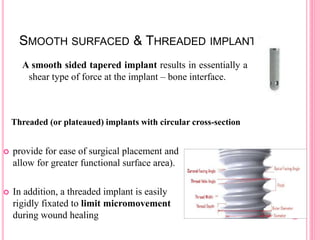
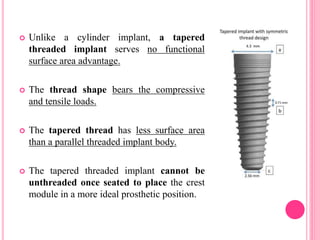
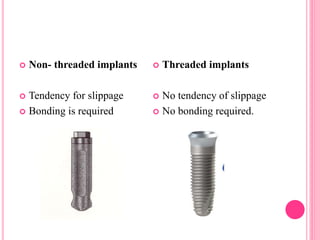
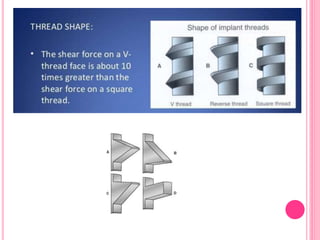
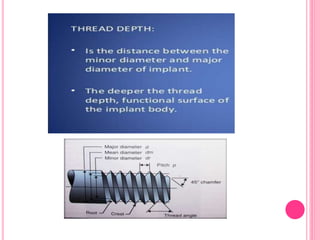
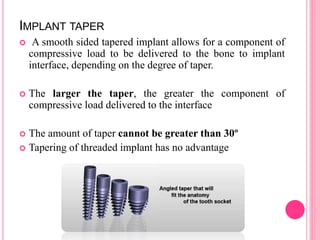
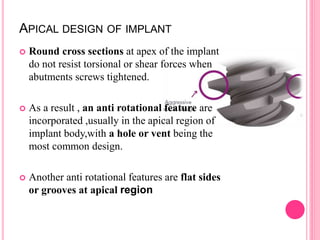
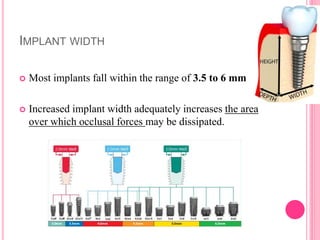
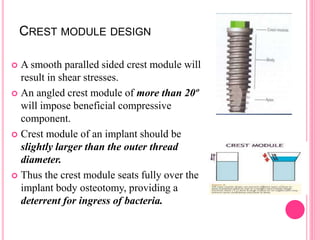
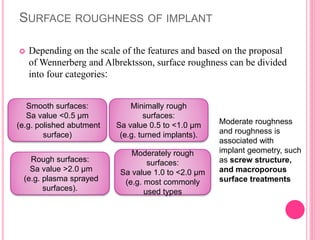
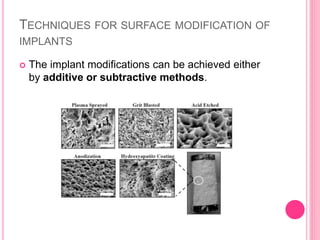
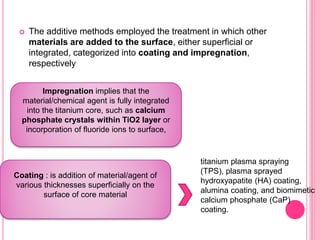
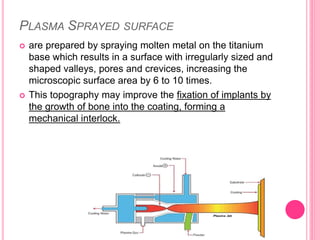
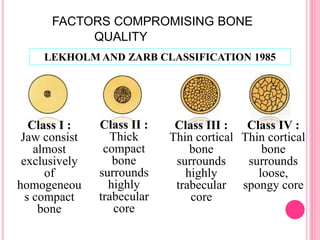
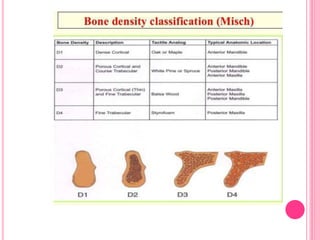
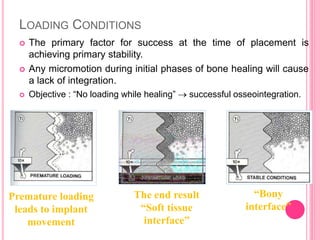
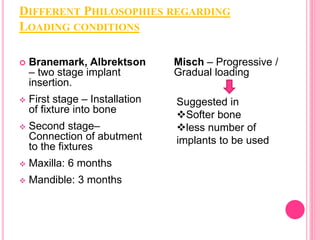
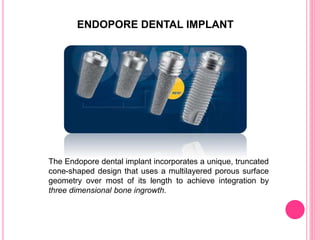
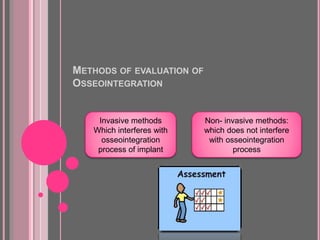
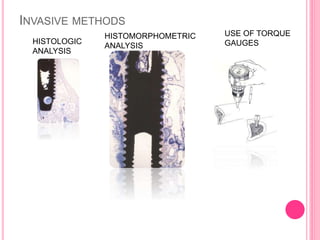
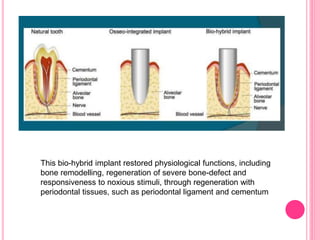
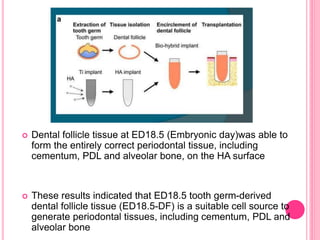
This document discusses osseointegration, which is the direct structural and functional connection between living bone and the surface of a load-carrying dental implant without intervening connective tissue. It covers the history, definitions, theories, mechanisms, and factors affecting osseointegration. The key points are that osseointegration was discovered by Branemark in the 1950s and involves new bone formation directly on implant surfaces through osteoconduction and remodeling over time to achieve a stable implant-bone interface. Factors like implant design, surface, material biocompatibility, and surgical technique influence the degree of osseointegration.