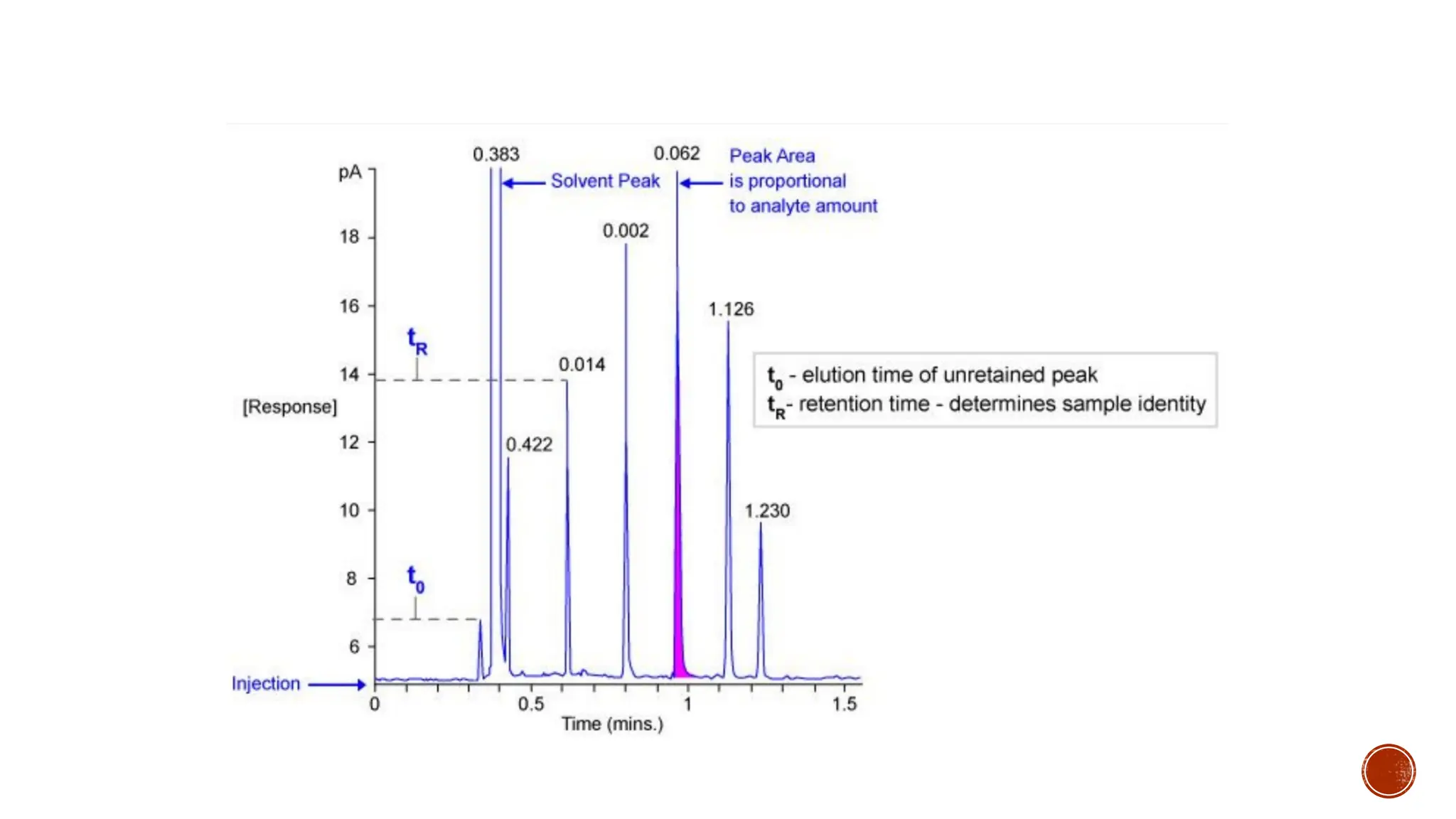
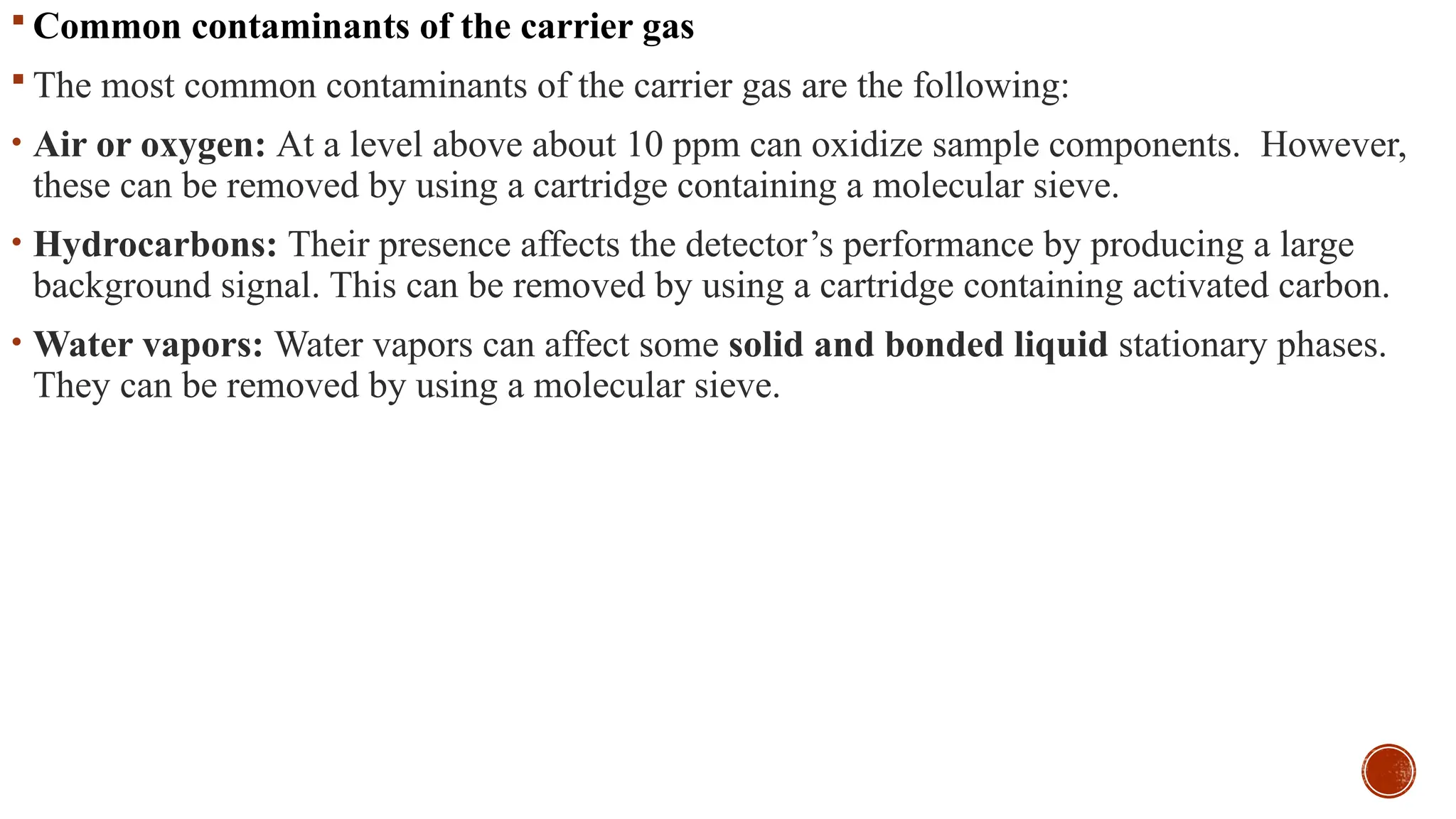
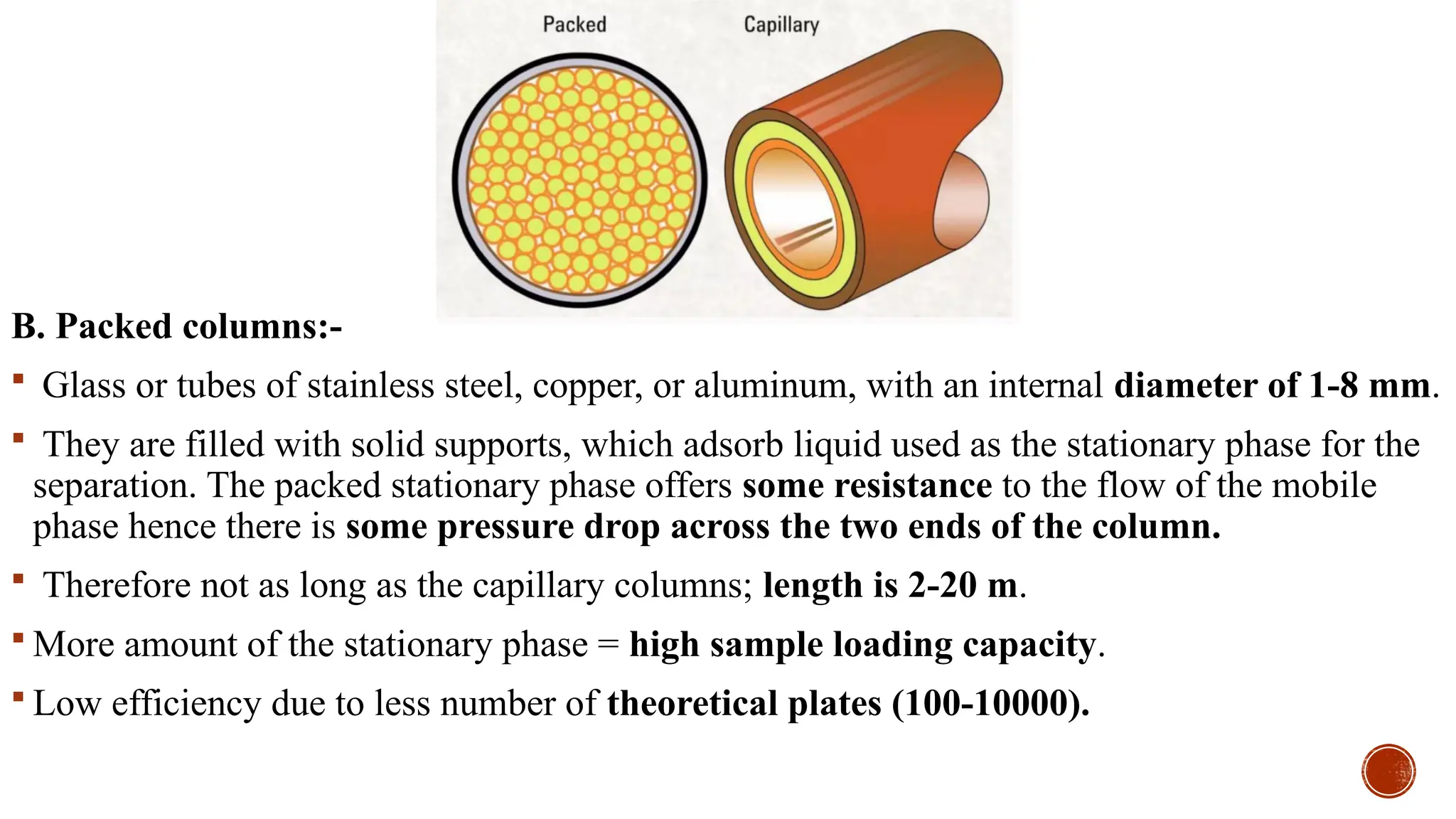
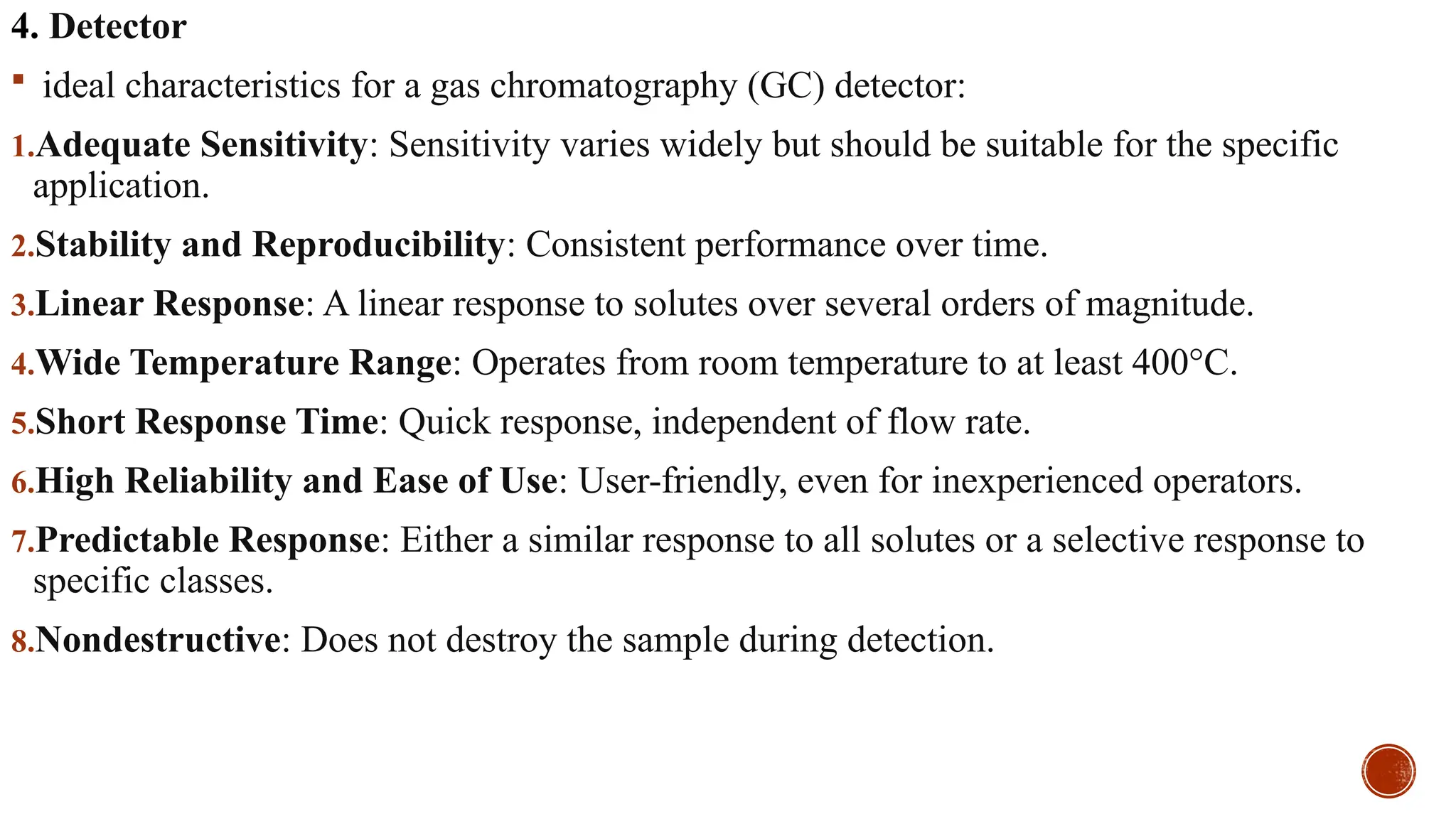
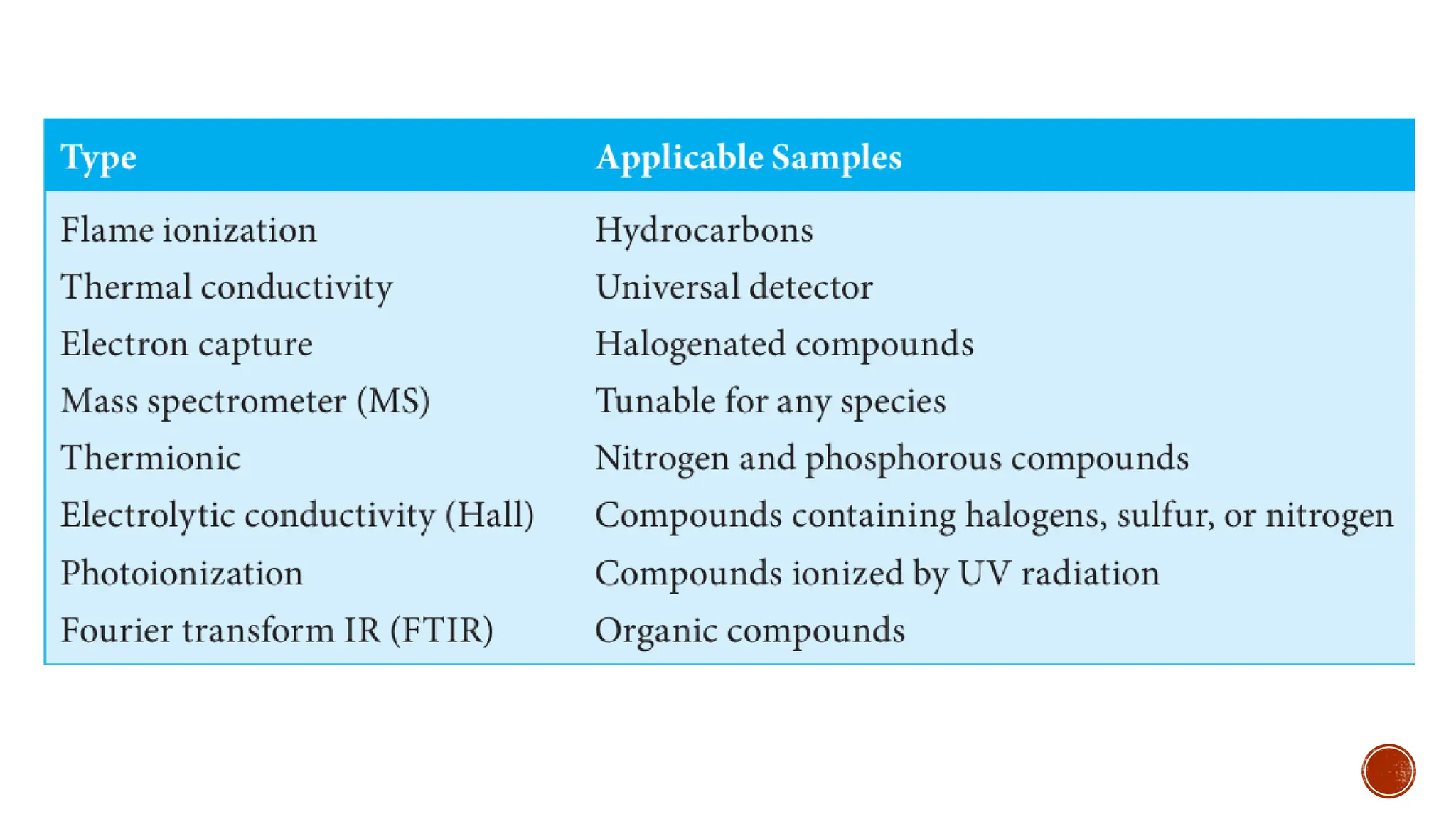
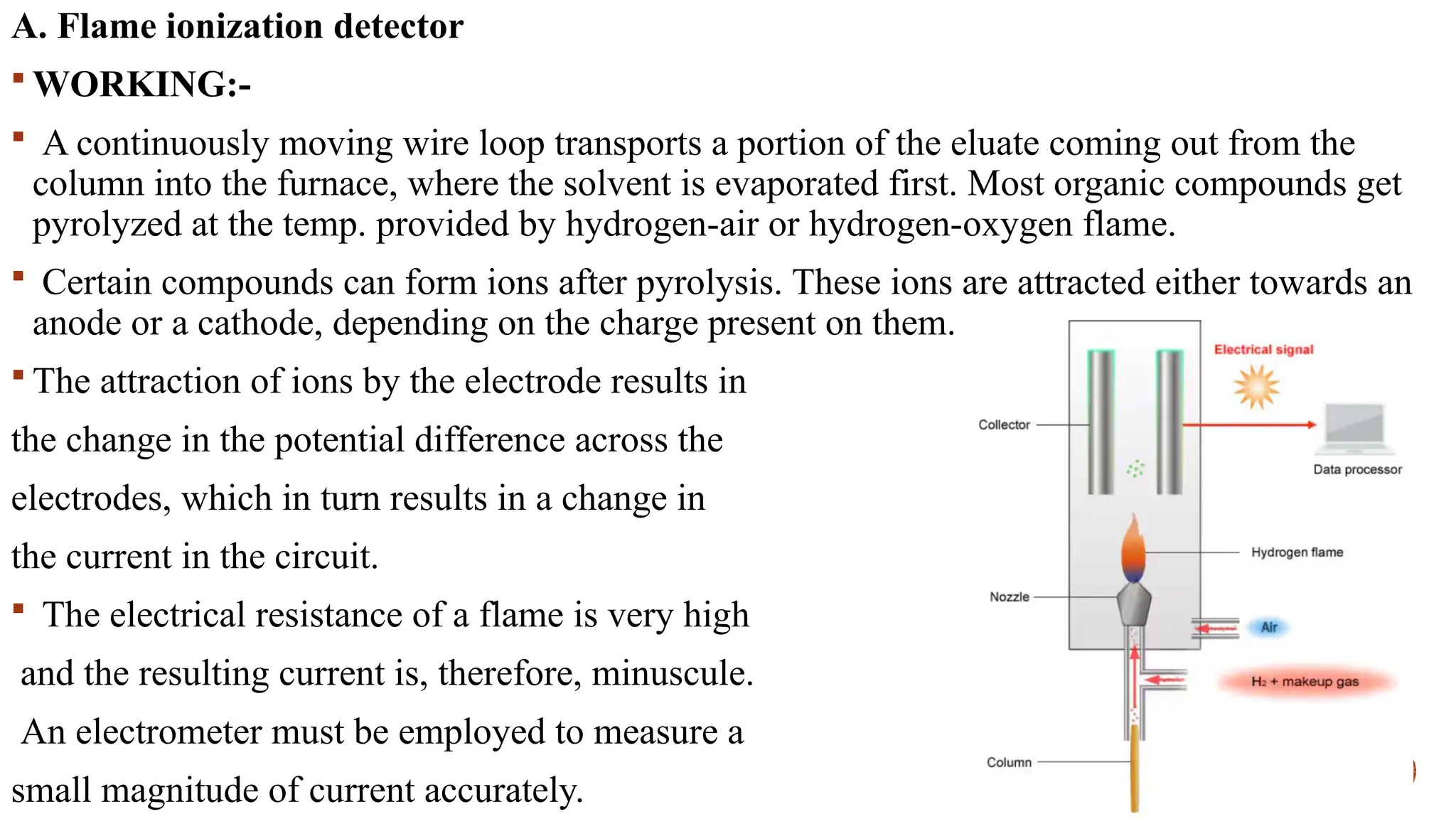
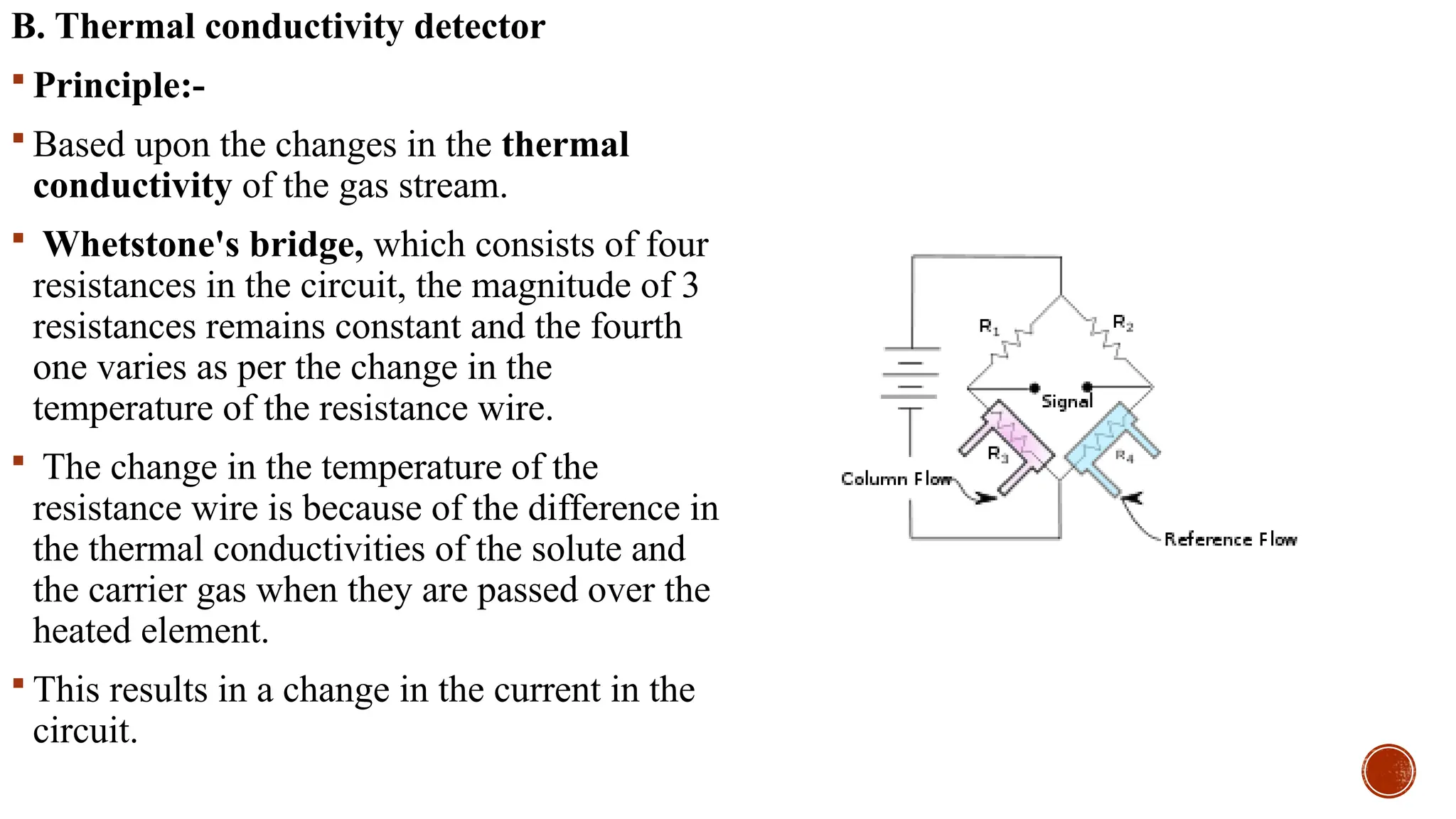
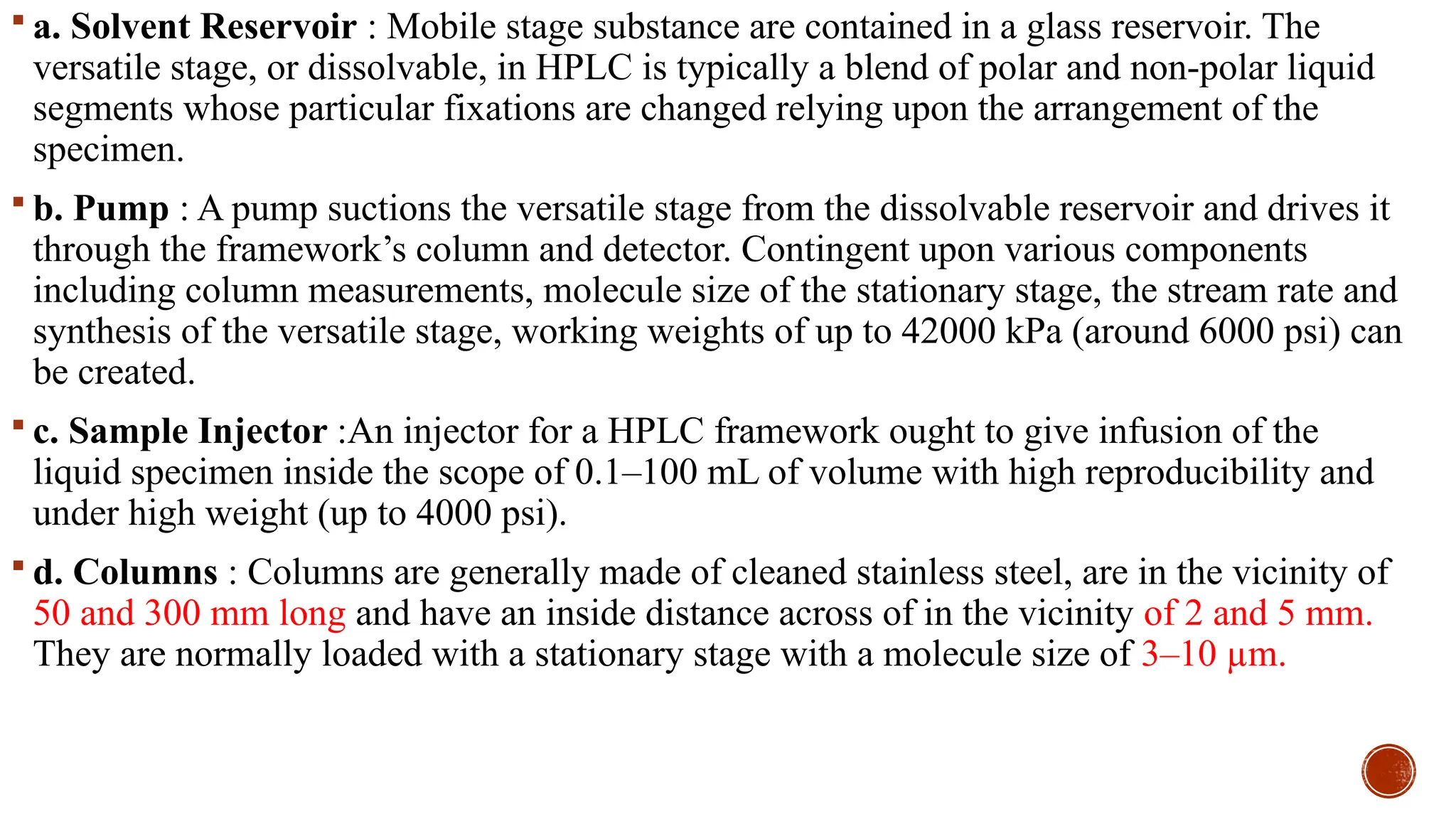
The document provides a comprehensive overview of gas chromatography, explaining its principles, instrumentation, advantages, and disadvantages. It details the various components involved, such as the carrier gas, sample injection system, columns, detectors, and their functions. Additionally, it discusses applications in different industries including food safety, environmental monitoring, and forensics, along with the concept of derivatization for non-volatile compounds.