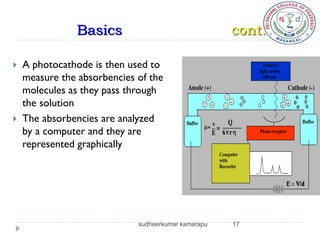
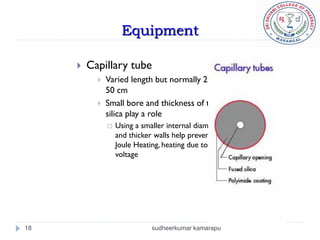
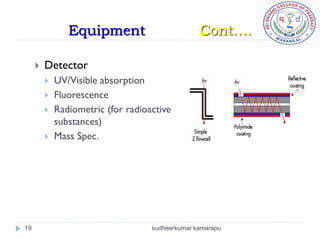
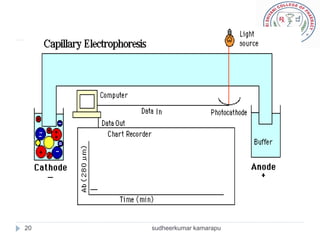
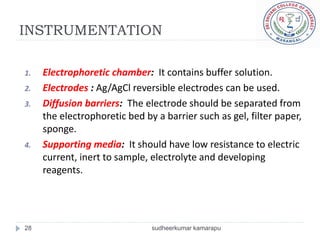
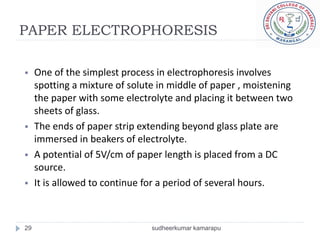
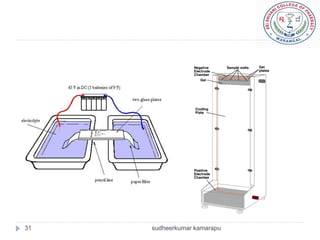
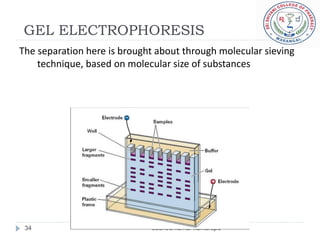
The document provides an overview of electrophoresis, including:
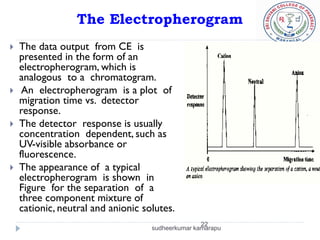
1) Electrophoresis is a separation technique where charged molecules migrate at different rates in an electric field, allowing separation.
2) It is used to separate biological substances like proteins, nucleic acids, and amino acids.
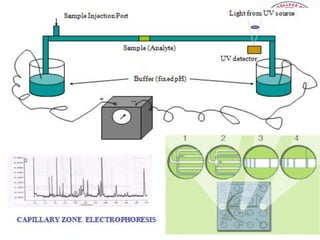
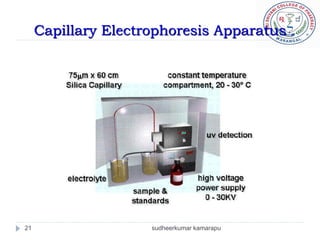
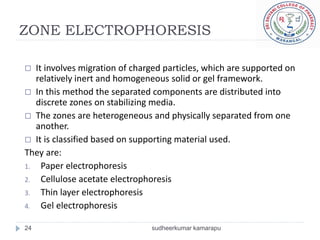
3) There are different electrophoresis methods including capillary electrophoresis, which uses narrow capillaries, and zone electrophoresis using papers, gels, or thin layers to support molecules.
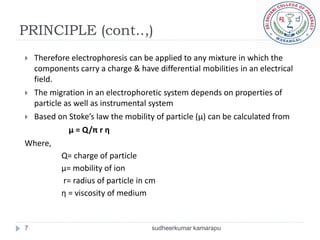
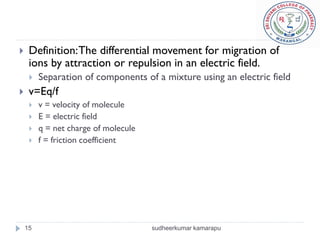
4) Factors like each molecule's charge, size, and the electric field strength determine their movement during electrophoresis.