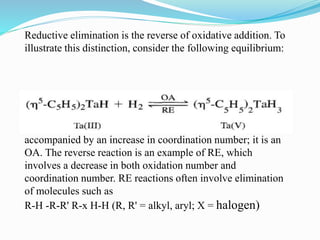
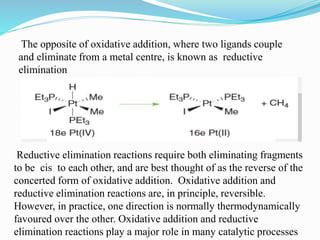
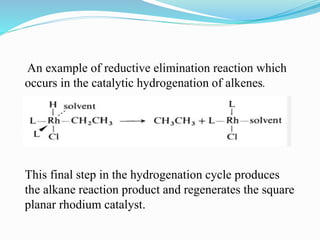
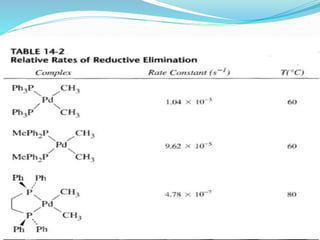
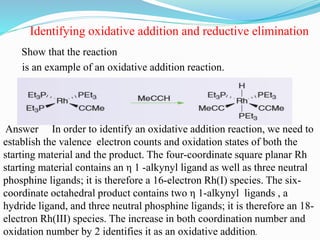
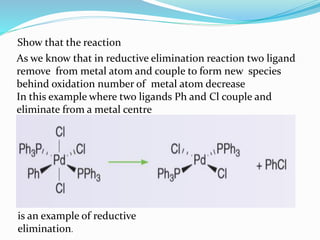
The document discusses reductive elimination reactions in organometallic chemistry, characterizing them as a step that results in a decrease in the oxidation state of a metal while forming new covalent bonds. It highlights the importance of these reactions in various catalytic processes, including hydrogenation and cross-coupling reactions, both in academia and industry. Additionally, the document explains the reversibility of oxidative addition and reductive elimination, providing examples and applications related to these reactions.