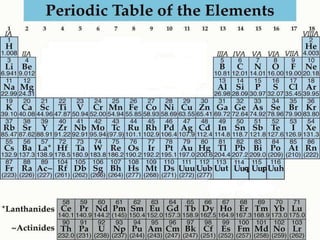
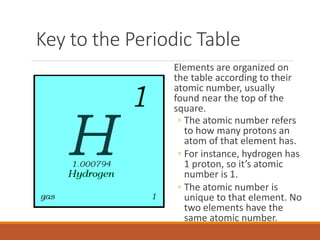
The document provides an overview of the periodic table of elements and key concepts related to elements and their organization. It discusses how elements are organized according to their atomic number and properties that can be determined from an element's position on the periodic table. Examples of different families of elements are provided, including their typical properties and valence electron configuration. Key terms like atomic number, atomic mass, and symbols are defined.