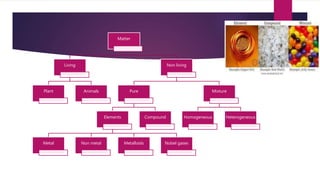
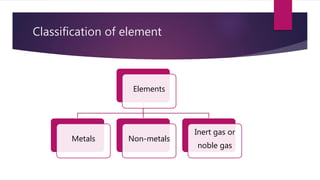
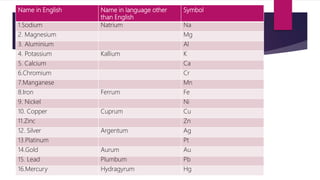
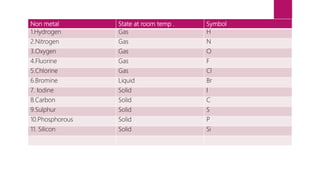
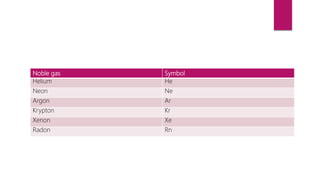
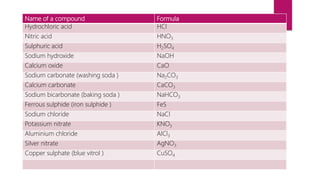
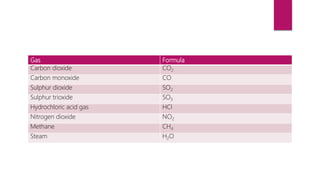
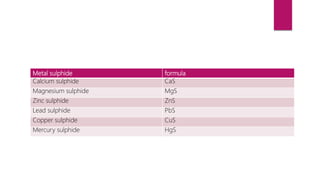
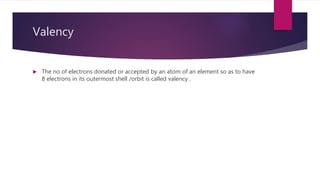
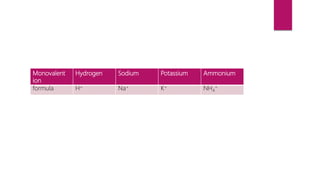
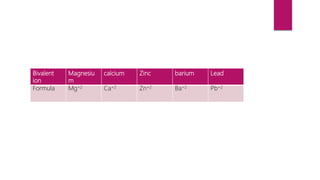
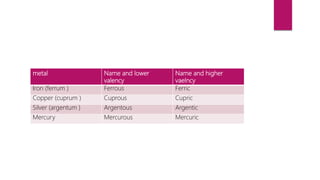
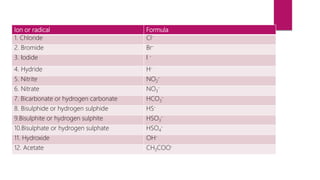
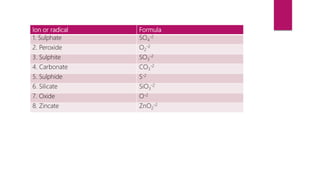
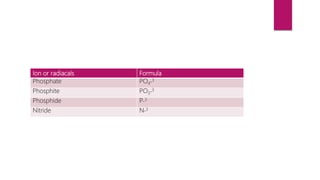
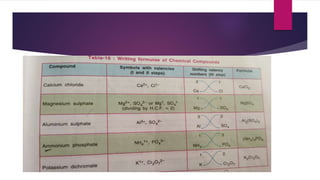
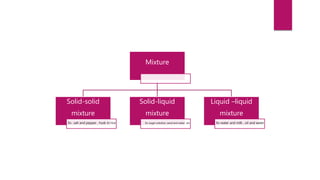
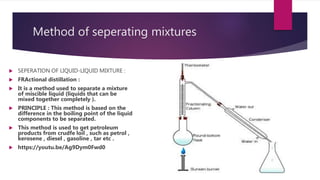
This document discusses elements, compounds, and mixtures. It defines elements as pure substances made of only one type of atom that cannot be separated into simpler substances. Compounds are made of two or more elements chemically bonded together in fixed ratios. Mixtures contain two or more substances mixed but not chemically combined. The document classifies elements and provides examples, discusses properties of compounds and how to write chemical formulas, and describes different types of mixtures and methods to separate them.