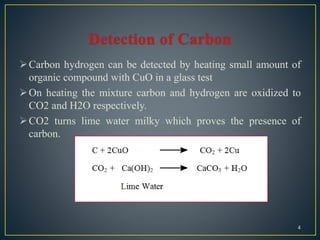
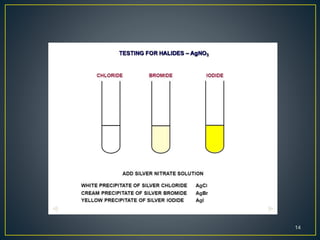
The document describes methods for detecting the elements carbon, hydrogen, nitrogen, sulfur, halogens, oxygen, phosphorus, and metals in organic compounds. Carbon and hydrogen can be detected by heating the organic compound with copper oxide and testing if carbon dioxide and water are produced. Nitrogen can be detected using Lassaigne's solution and testing for Prussian blue color. Sulfur can be detected using Lassaigne's solution and testing if it turns lead acetate paper black. Halogens can be detected using Lassaigne's solution and silver nitrate. Oxygen is generally inferred through indirect methods like detecting functional groups or determining percentages of other elements. Phosphorus can be detected by oxidizing it to