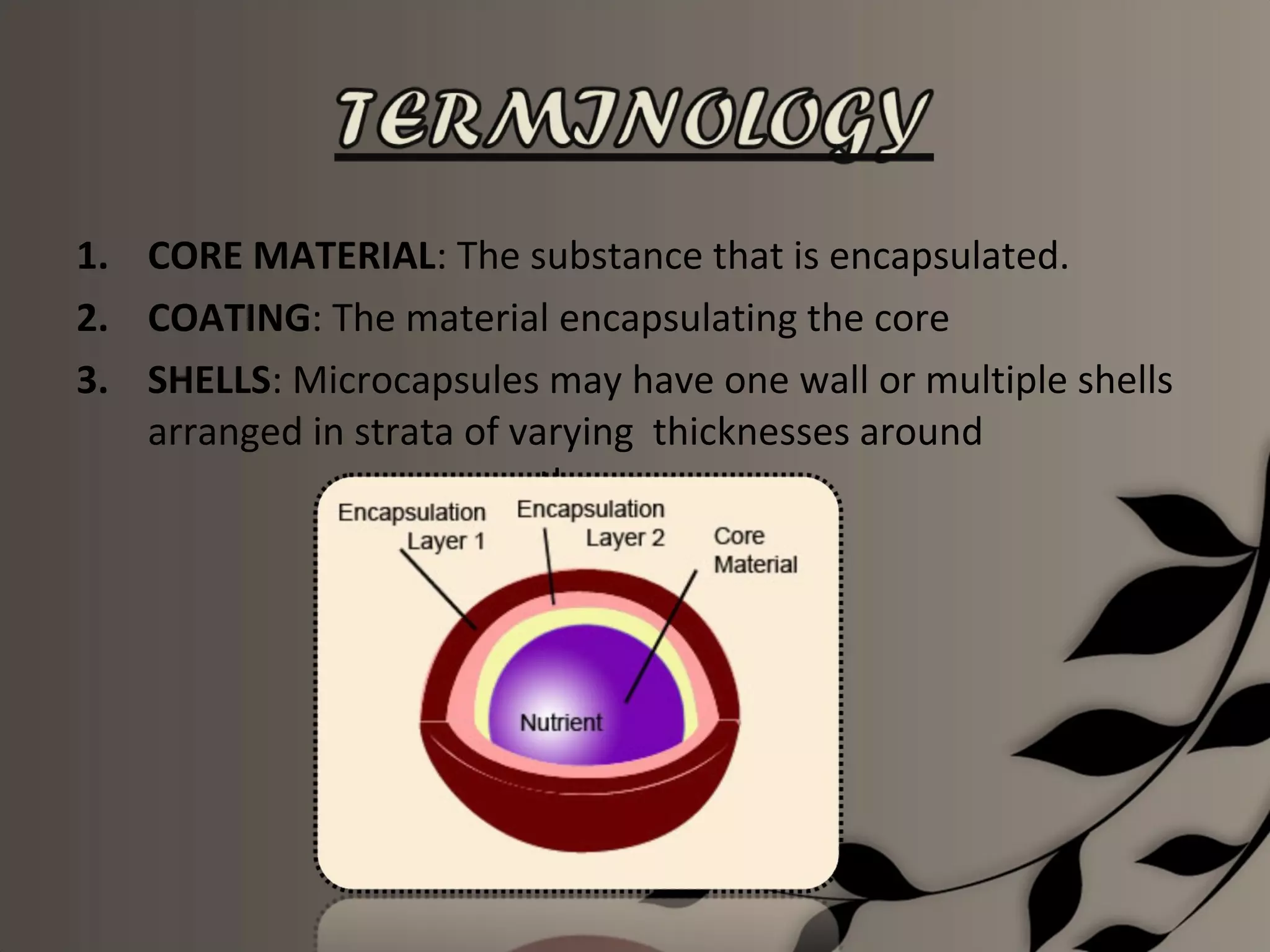
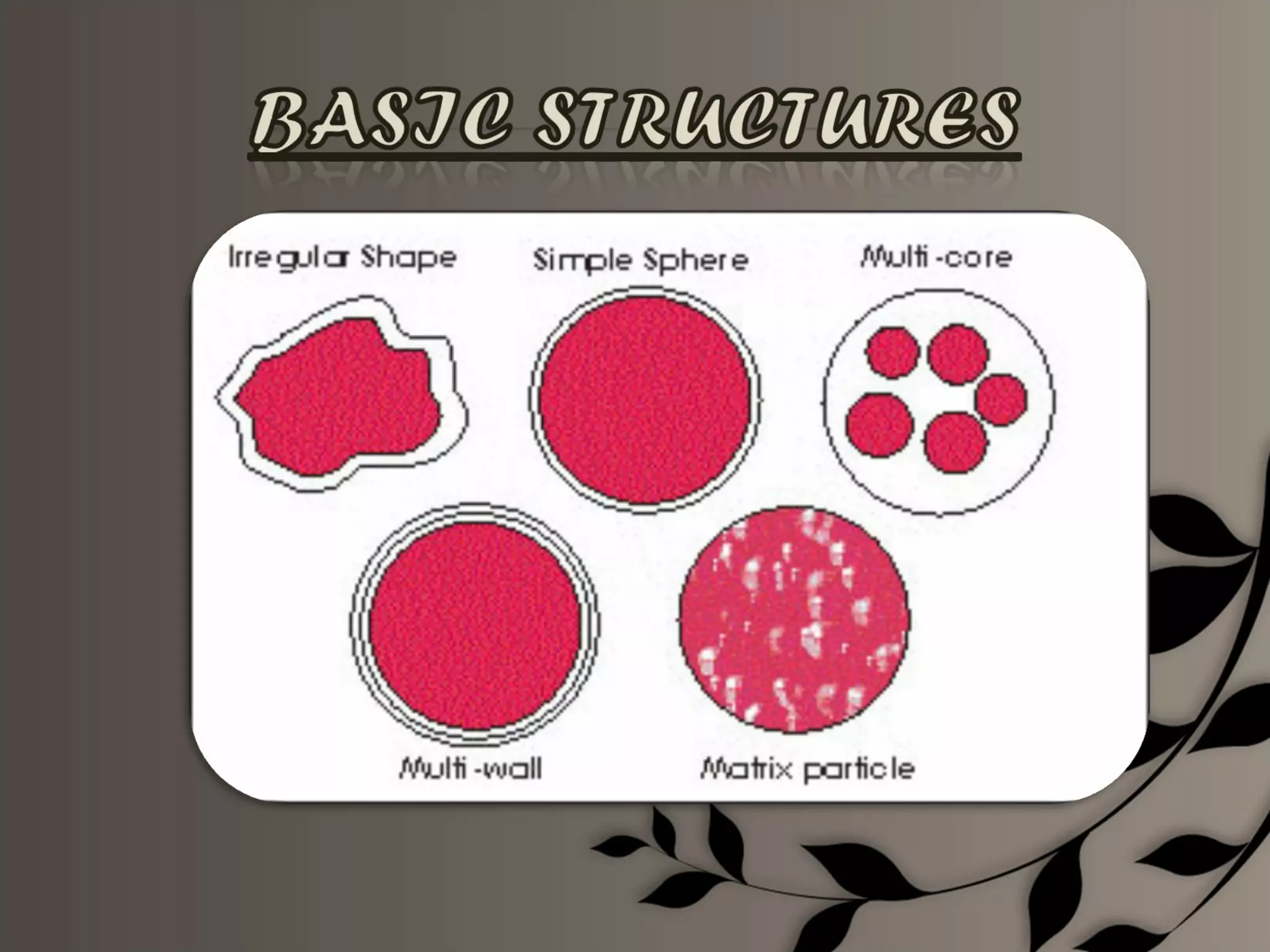
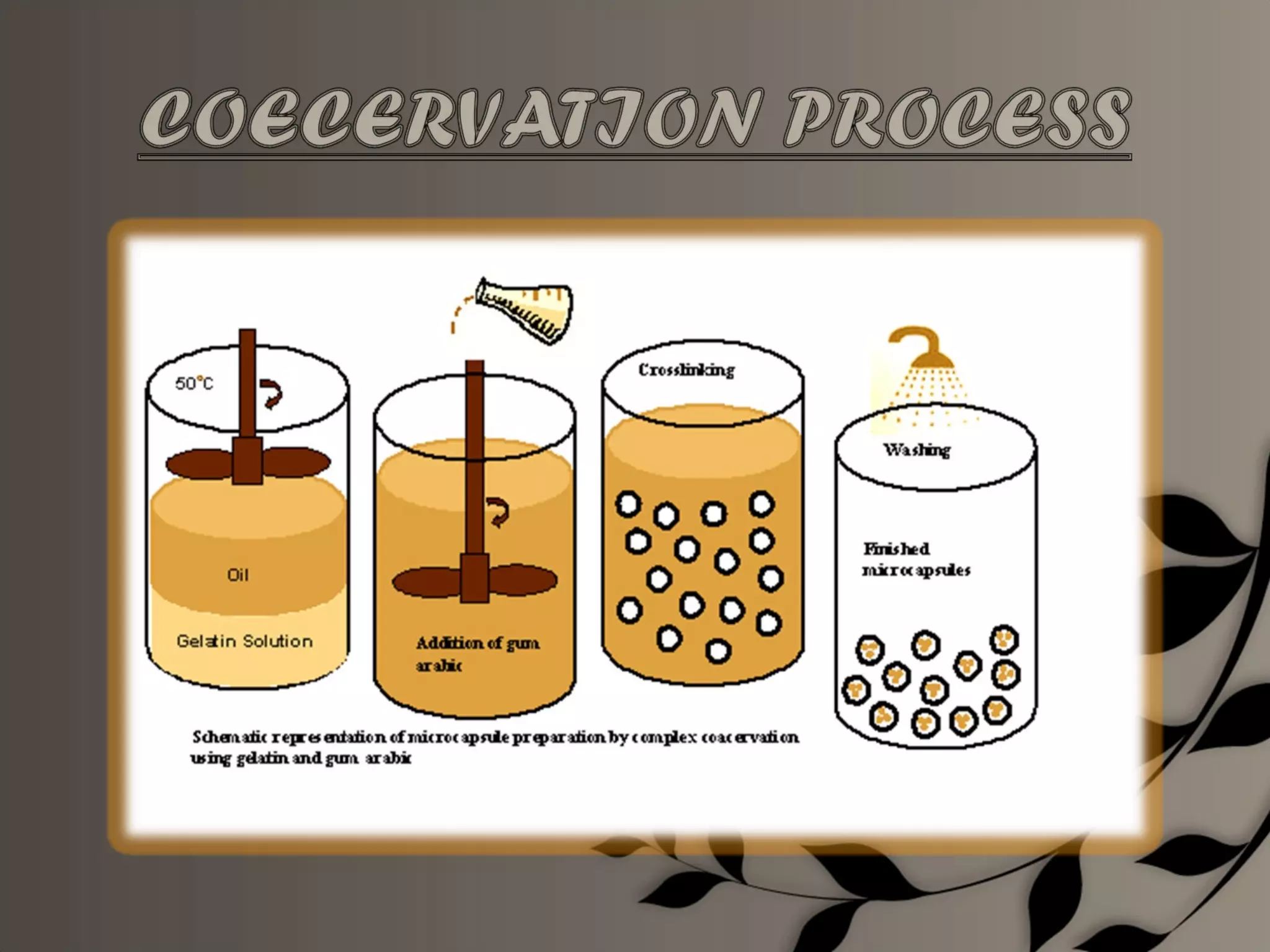
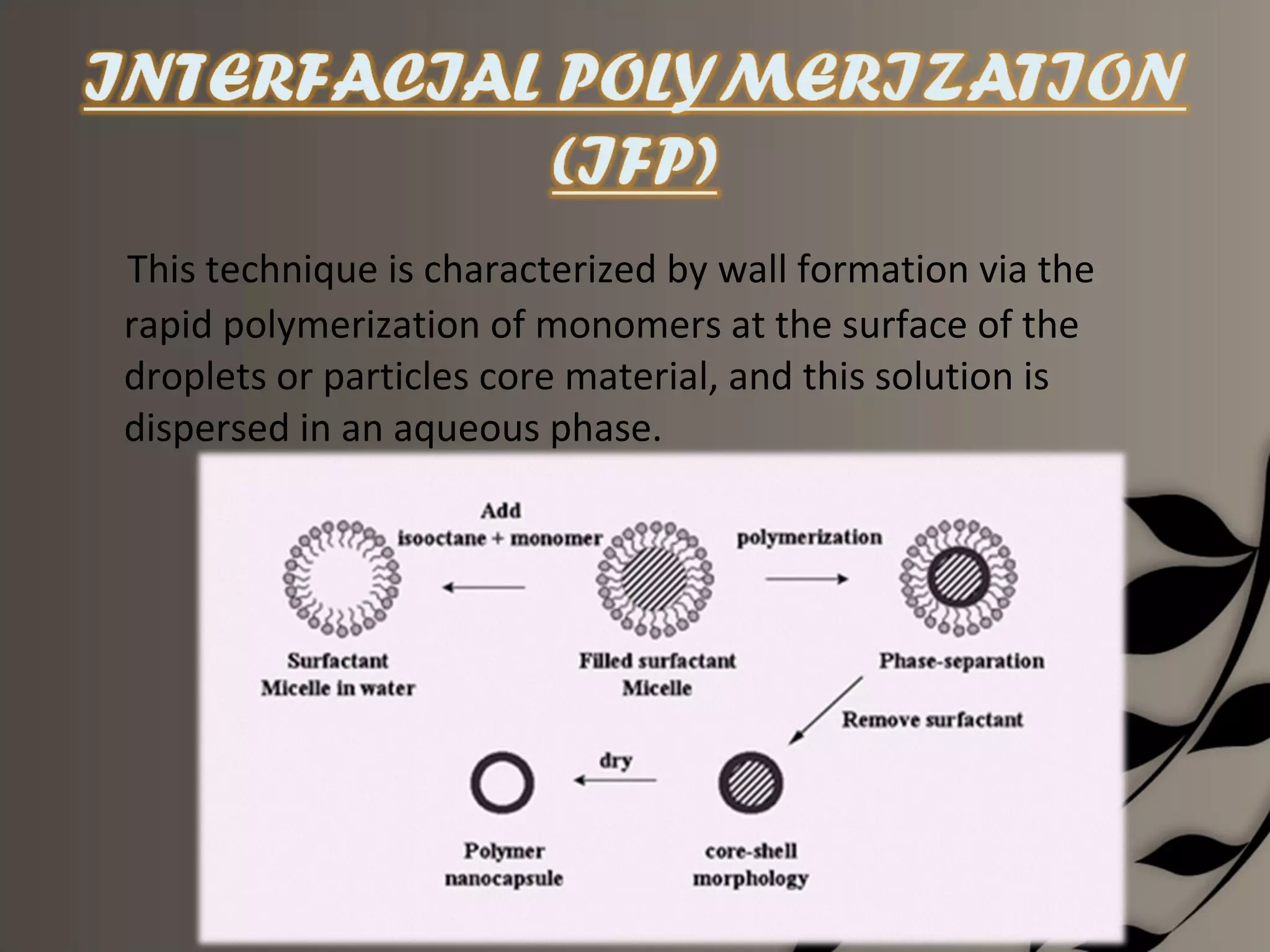
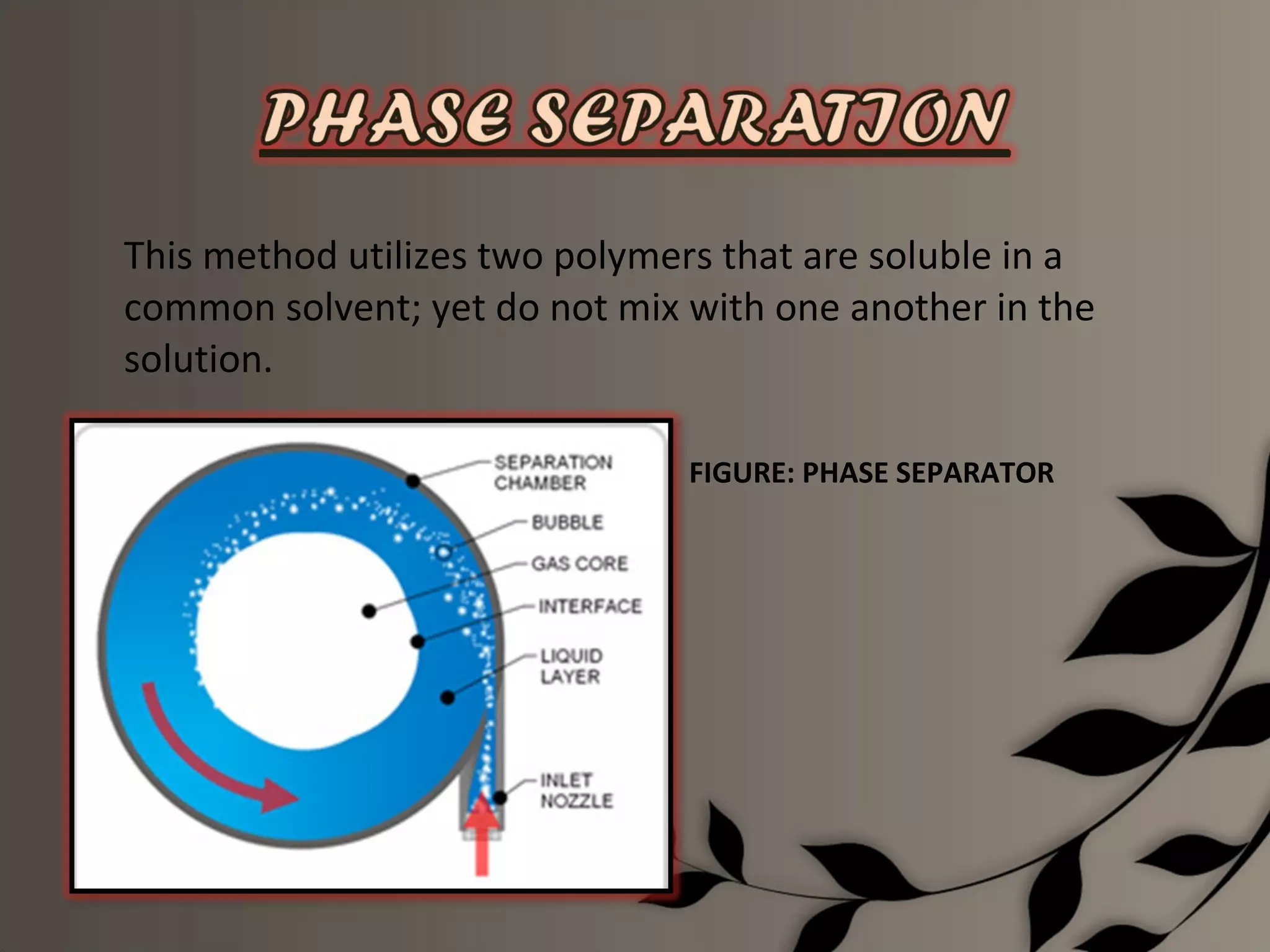
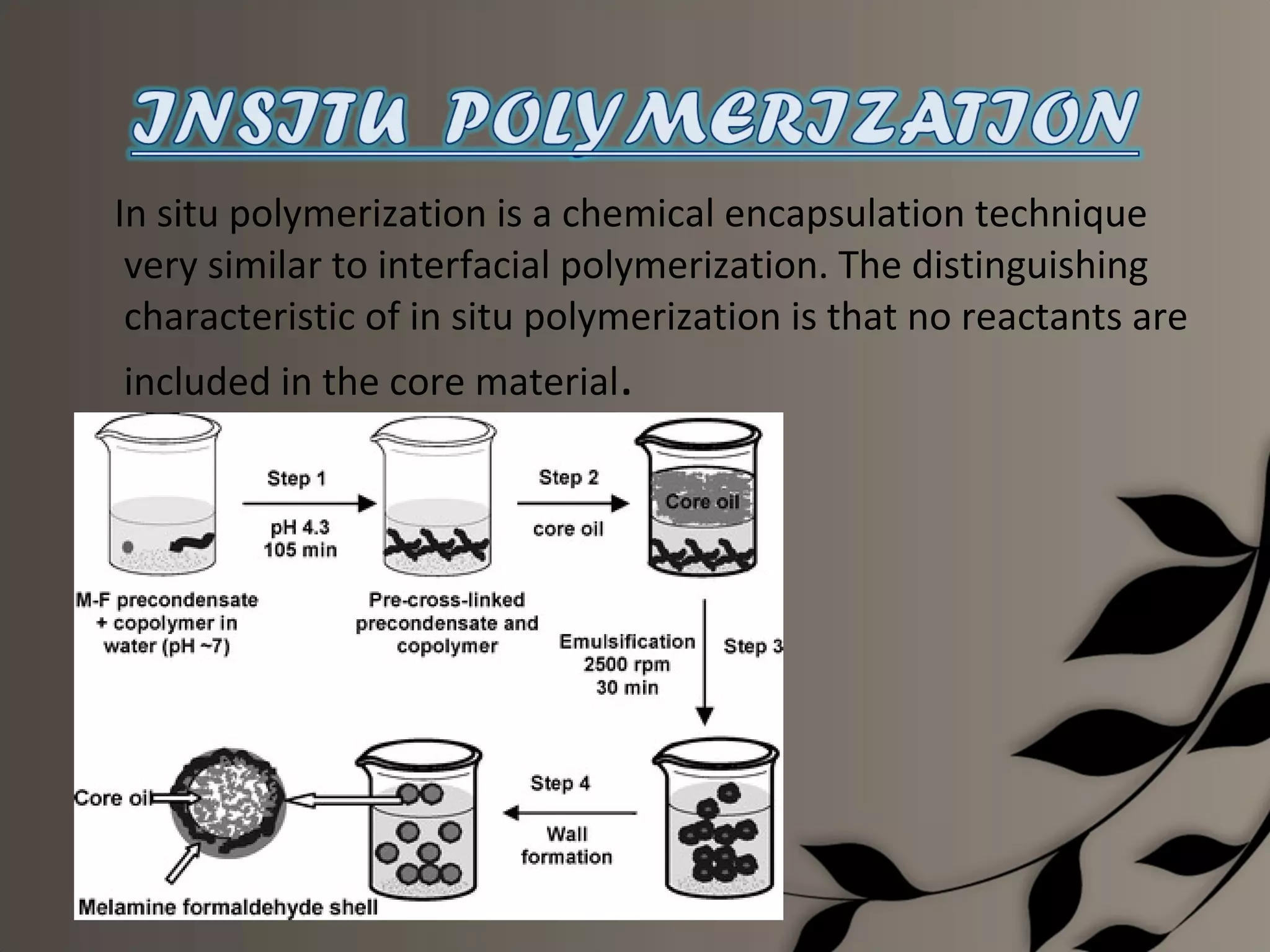
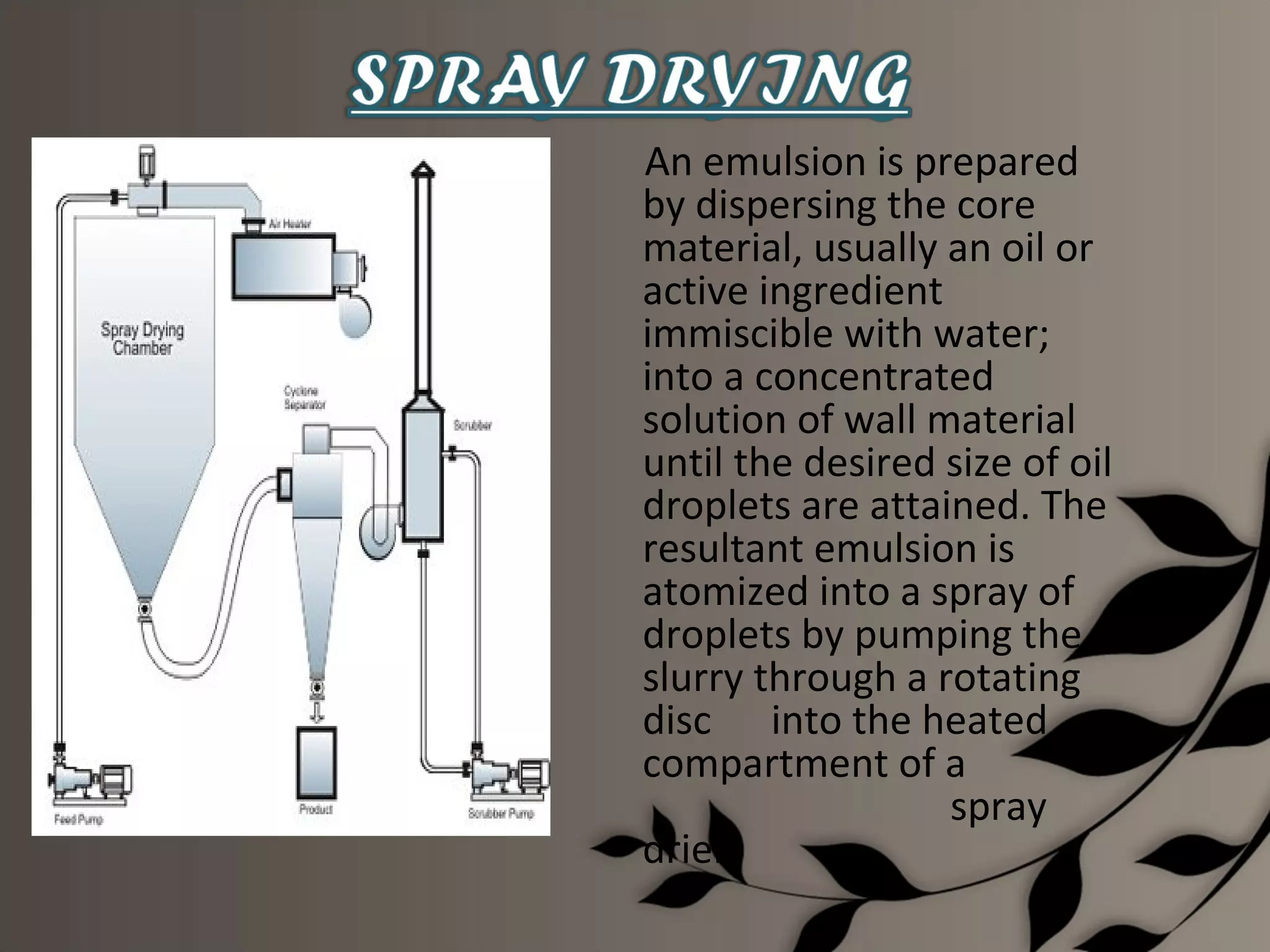
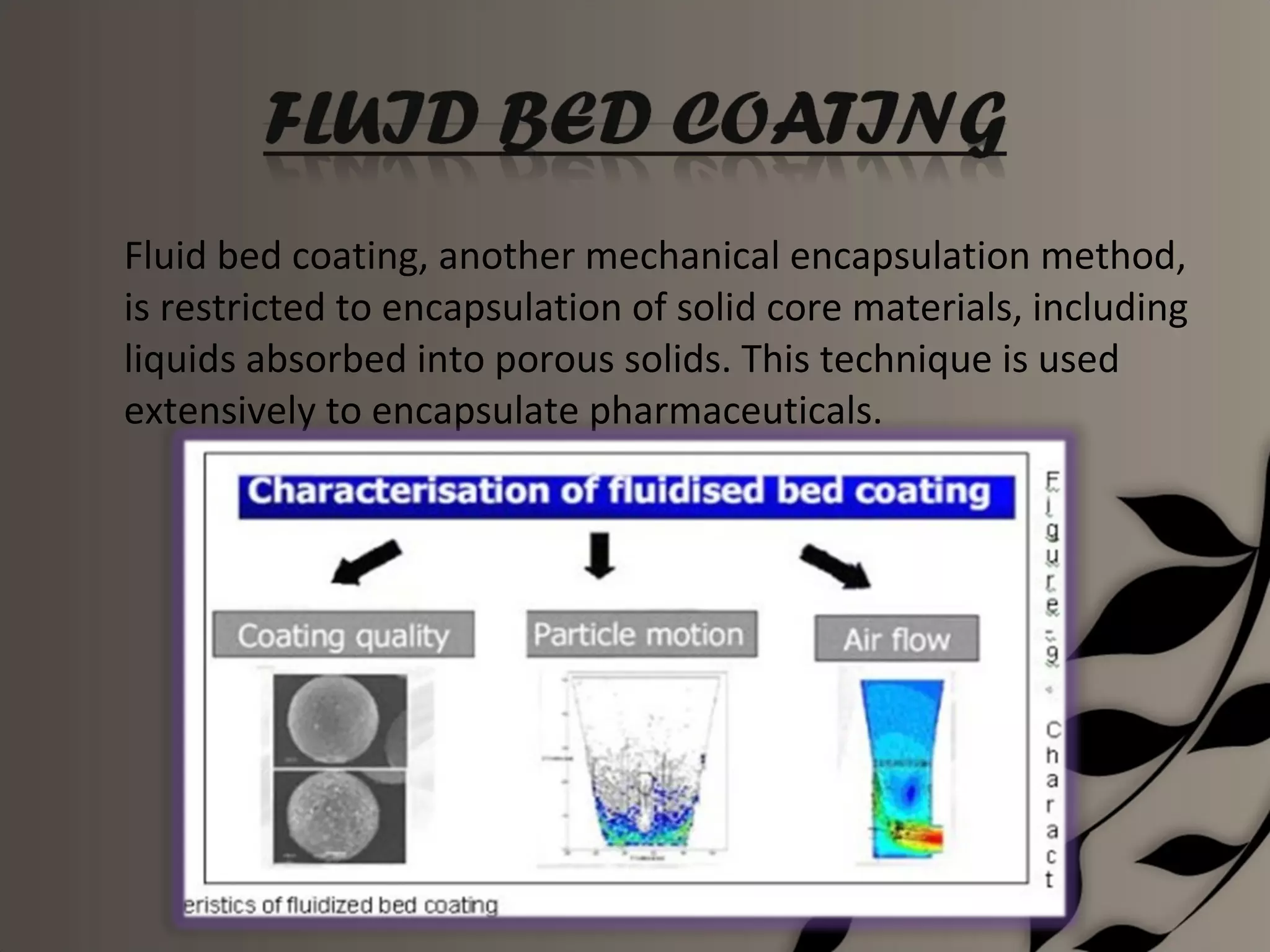
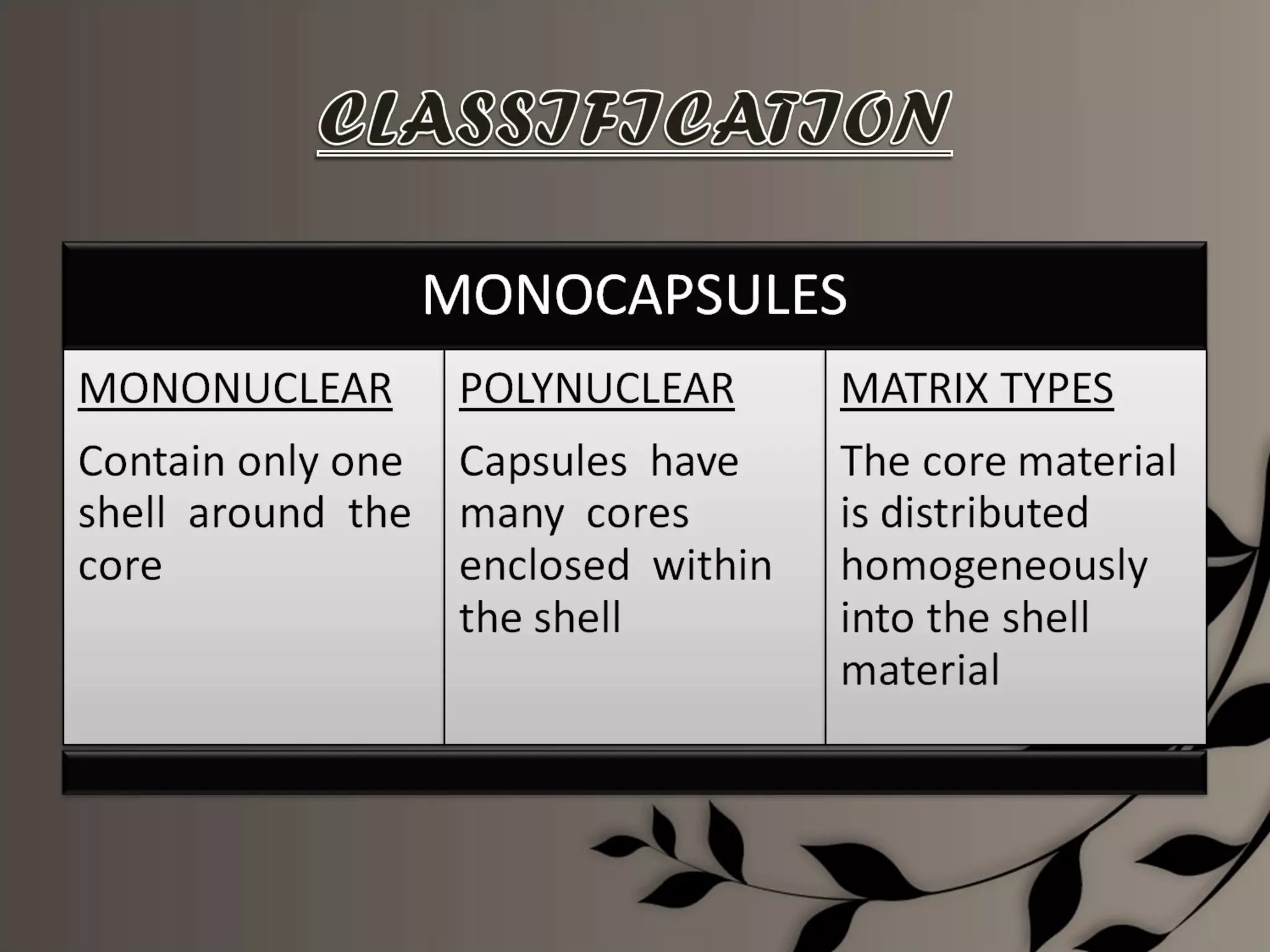
Microencapsulation is a process of enclosing core materials within microcapsules, utilizing both chemical and physical methods to control the release and protect the core from external factors. Applications range from pharmaceuticals to food products, improving stability, masking tastes, and enhancing handling properties. Despite advancements, challenges remain in selecting appropriate materials and techniques for effective microencapsulation.