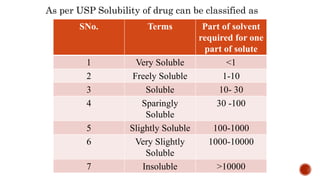
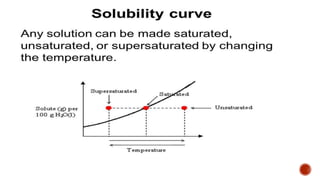
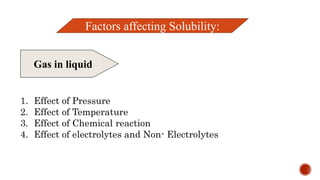
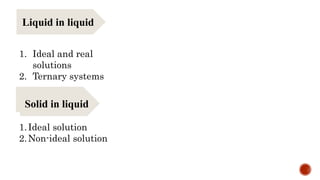
The document discusses solubility definitions, classifications, and its importance in pharmaceutical sciences. Solubility affects drug absorption, formulation development, dosage accuracy, stability, and bioavailability, and is influenced by various factors including temperature and pressure. It highlights the necessity of understanding solubility for effective drug design and delivery.