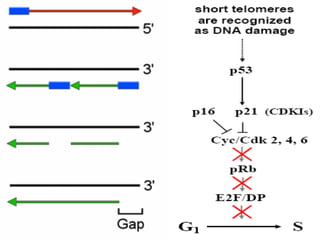
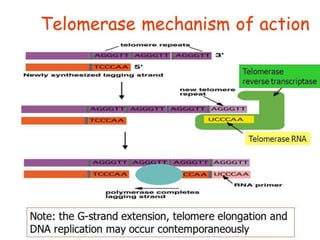
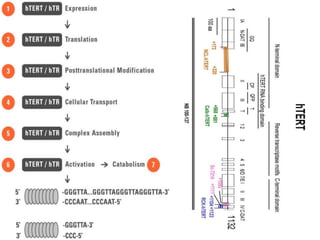
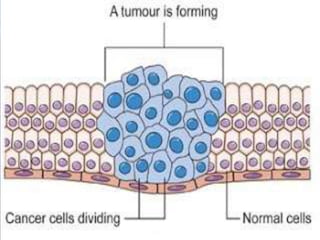
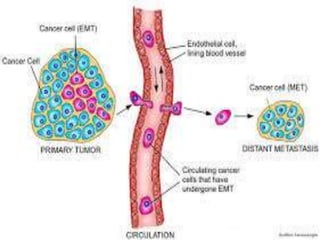
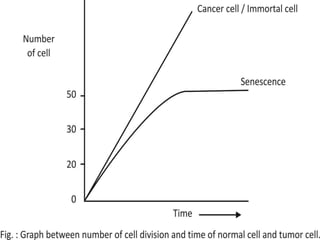
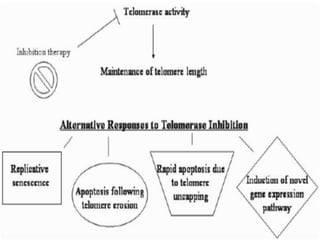
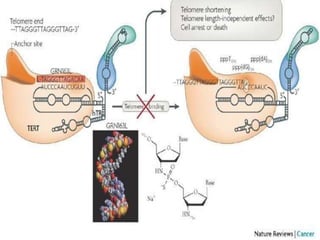
Telomeres and the telomerase enzyme play important roles in cellular aging and cancer development. In cancer cells, telomerase is abnormally overexpressed, allowing cells to overcome the Hayflick limit and become immortal. The telomerase enzyme maintains telomere length through its reverse transcriptase activity, using an RNA component as a template. Several approaches are being explored to develop telomerase inhibitors as anti-cancer drugs, including targeting the enzyme's components or using them together with conventional chemotherapy to increase treatment effectiveness against various cancer types. By inhibiting telomerase, cells would be unable to maintain telomere length and would eventually senesce or die.


![What is Telomerase
Telomeric Sequence is Maintained by a special Enzyme Called TELOMERASE.
Telomerase, also called terminal transferase,[1] is a ribonucleoprotein that adds
a species-dependent telomere repeat sequence to the 3' end of telomeres. A
telomere is a region of repetitive sequences at each end
of eukaryotic chromosomes in most eukaryotes. Telomeres protect the end of
the chromosome from DNA damage or from fusion with neighboring
chromosomes.
Telomerase is a reverse transcriptase enzyme that carries its own RNA molecule
which is used as a template when it elongates telomeres. Telomerase is active
in normal stem cells and most cancer cells, but is normally absent from, or at
very low levels in, most somatic cells.](https://image.slidesharecdn.com/telomere-180516192055/85/Telomere-and-telomerase-inbitors-6-320.jpg)

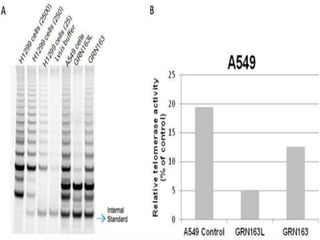

![TELOMERASE INHIBITORS WITH CONVENTIONAL
THERAPY
• As reviewed here, telomerase inhibitors have been shown
to be effective by themselves as potentially valuable
therapeutic agents. However, their greatest use may come
not as stand-alone pharmaceuticals but as part of a
coordinated treatment strategy in conjunction with
standard treatments including various chemotherapeutics
and radiation therapy. Several of the studies cited in this
review demonstrated success with their telomerase
inhibitors both alone and with synergistic effects when
combined with other chemotherapeutics. The method of
action of the various chemotherapeutic agents include
topoisomerase inhibitors
'-O-methoxyethyl RNA Cisplatin/carboplatin Synergistic effect
Antisense-hTR Paclitaxel
Significant increase in
sensitivity
Antisense-hTR Cisplatin Increase in sensitivity
DN-hTERT
Cisplatin/taxanes/etoposi
de
Increase in induction of
apoptosis
DN-hTERT Daunorubicin Increase in apoptosis
Ribozyme-hTERT Doxorubicin Increase in sensitivity
RNAi-hTERT
Topoisomerase
inhibitors/bleomycin/radi
ation
Increase in sensitivity
hTR-NAT [131
I]MIBG Induced uptake
AZT Paclitaxel
Increased activity and
effect](https://image.slidesharecdn.com/telomere-180516192055/85/Telomere-and-telomerase-inbitors-17-320.jpg)







![REFFERENCES
• shikawa F. Regulation mechanisms of mammalian telomerase. A
review. Biochemistry (Mosc) 1997;62:1332–1337. [PubMed]
• 2. Weinrich SL, Pruzan R, Ma L, Ouellette M, Tesmer VM, Holt
SE, Bodnar AG, Lichtsteiner S, Kim NW, Trager JB, Taylor RD,
Carlos R, Andrews WH, Wright WE, Shay JW, Harley CB, Morin
GB. Reconstitution of human telomerase with the template
RNA component hTR and the catalytic protein subunit
hTRT. Nat. Genet. 1997;17:498–502. [PubMed]
• 3. Beattie TL, Zhou W, Robinson MO, Harrington L.
Reconstitution of human telomerase activity in vitro. Curr.
Biol. 1998;29:177–180. [PubMed]
• 4. Shay JW, Bacchetti S. A survey of telomerase activity in
human cancer. Eur. J. Cancer. 1997;33:787–791. [PubMed]](https://image.slidesharecdn.com/telomere-180516192055/85/Telomere-and-telomerase-inbitors-26-320.jpg)
