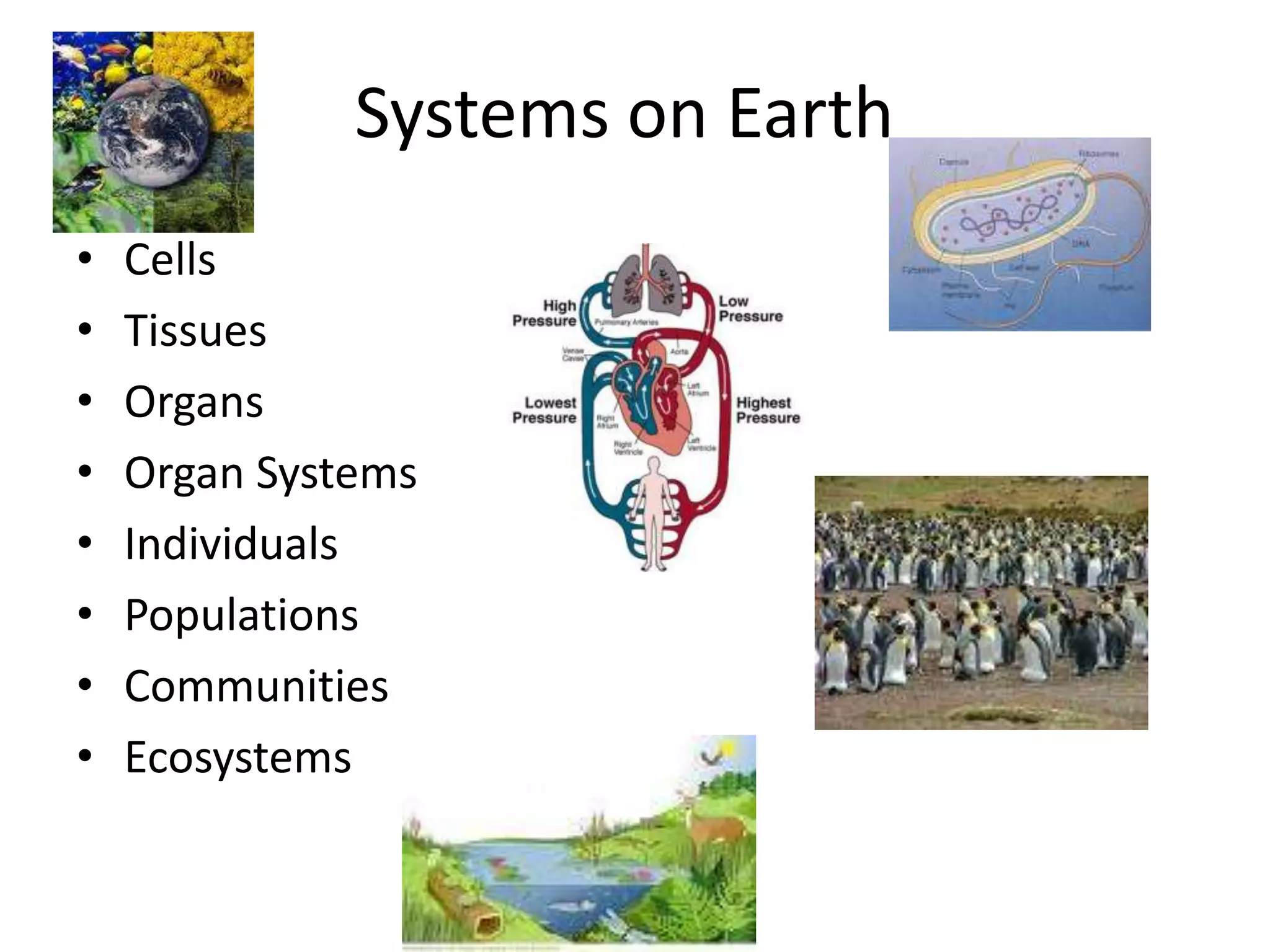
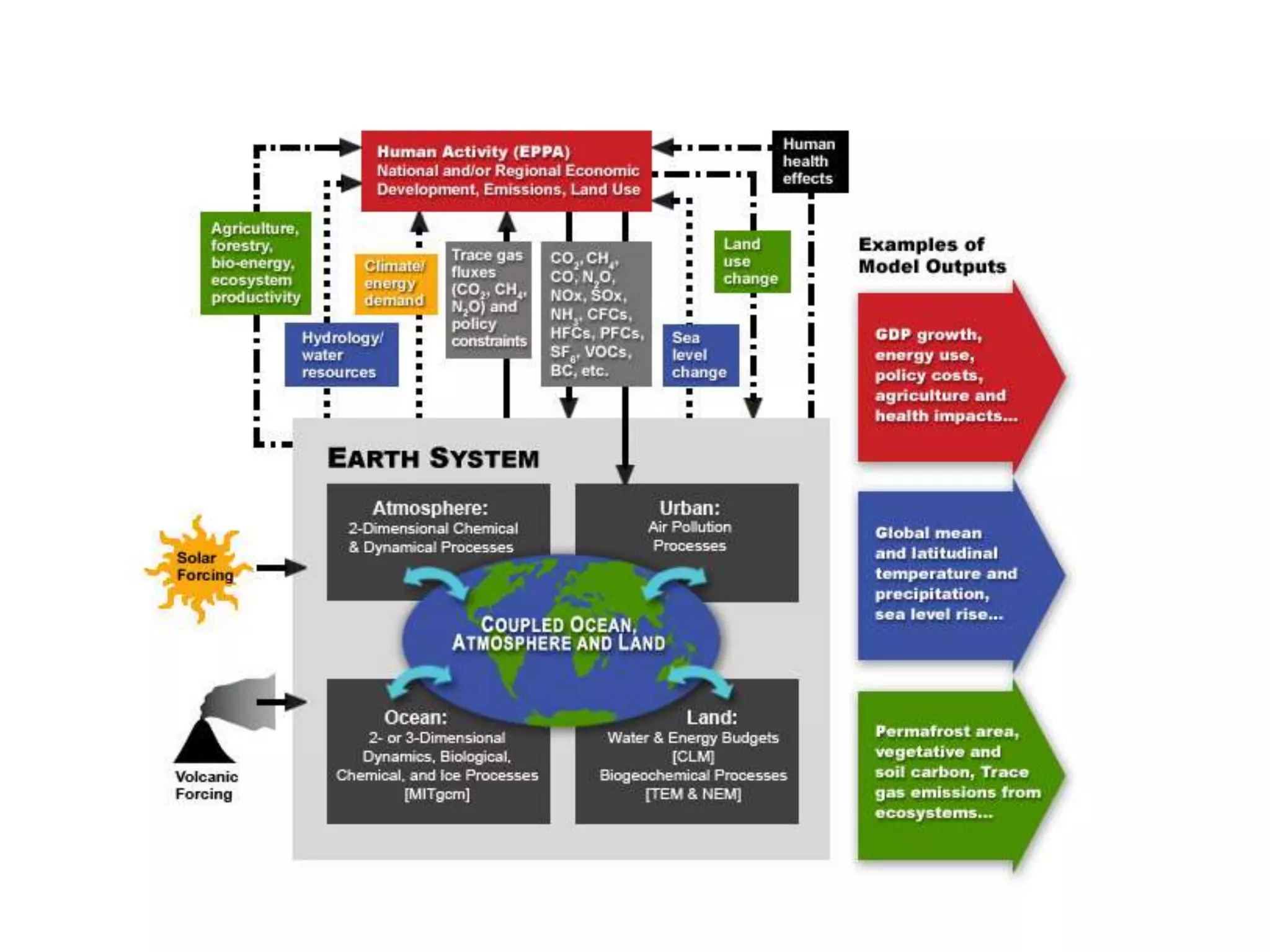
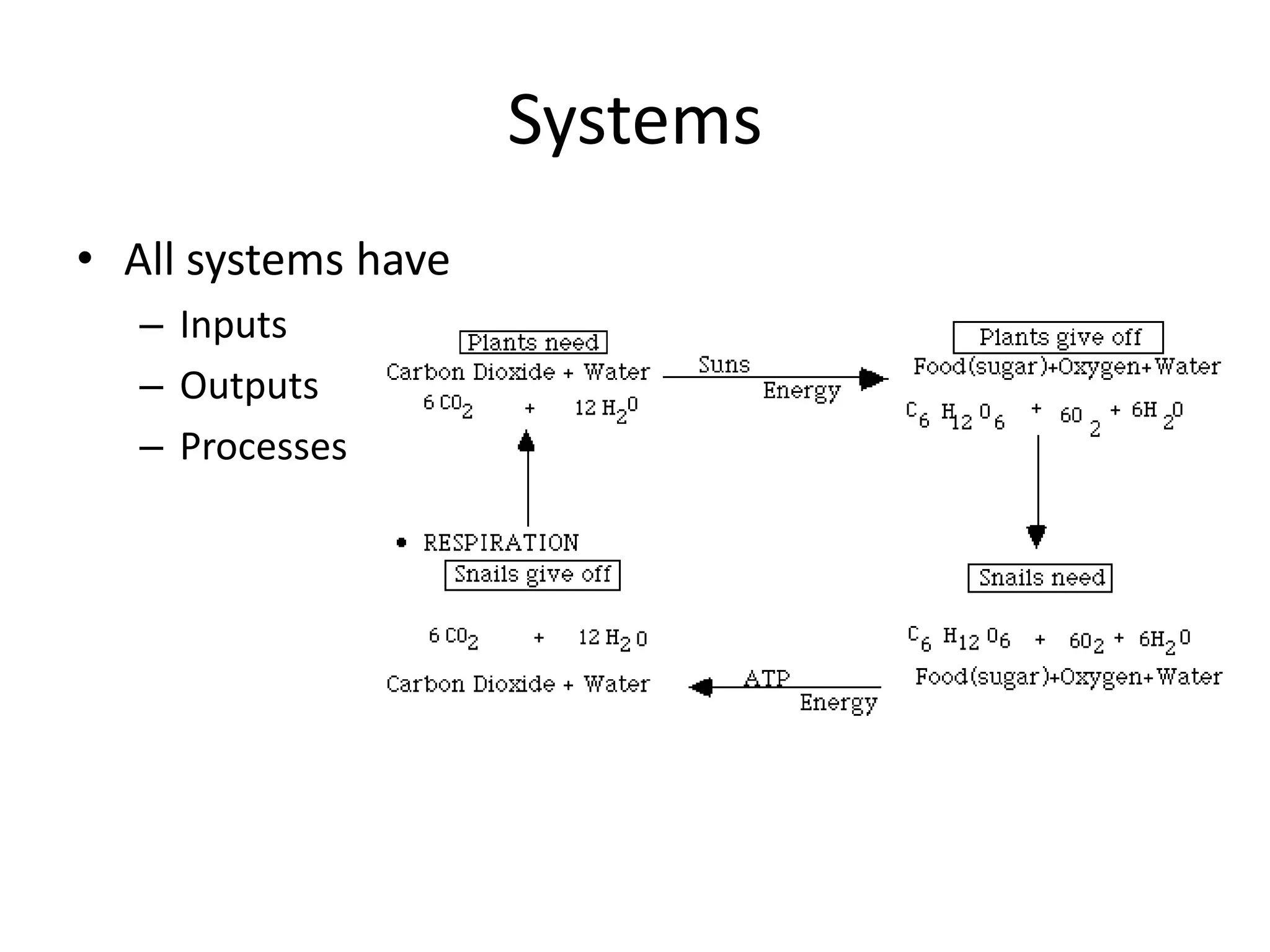
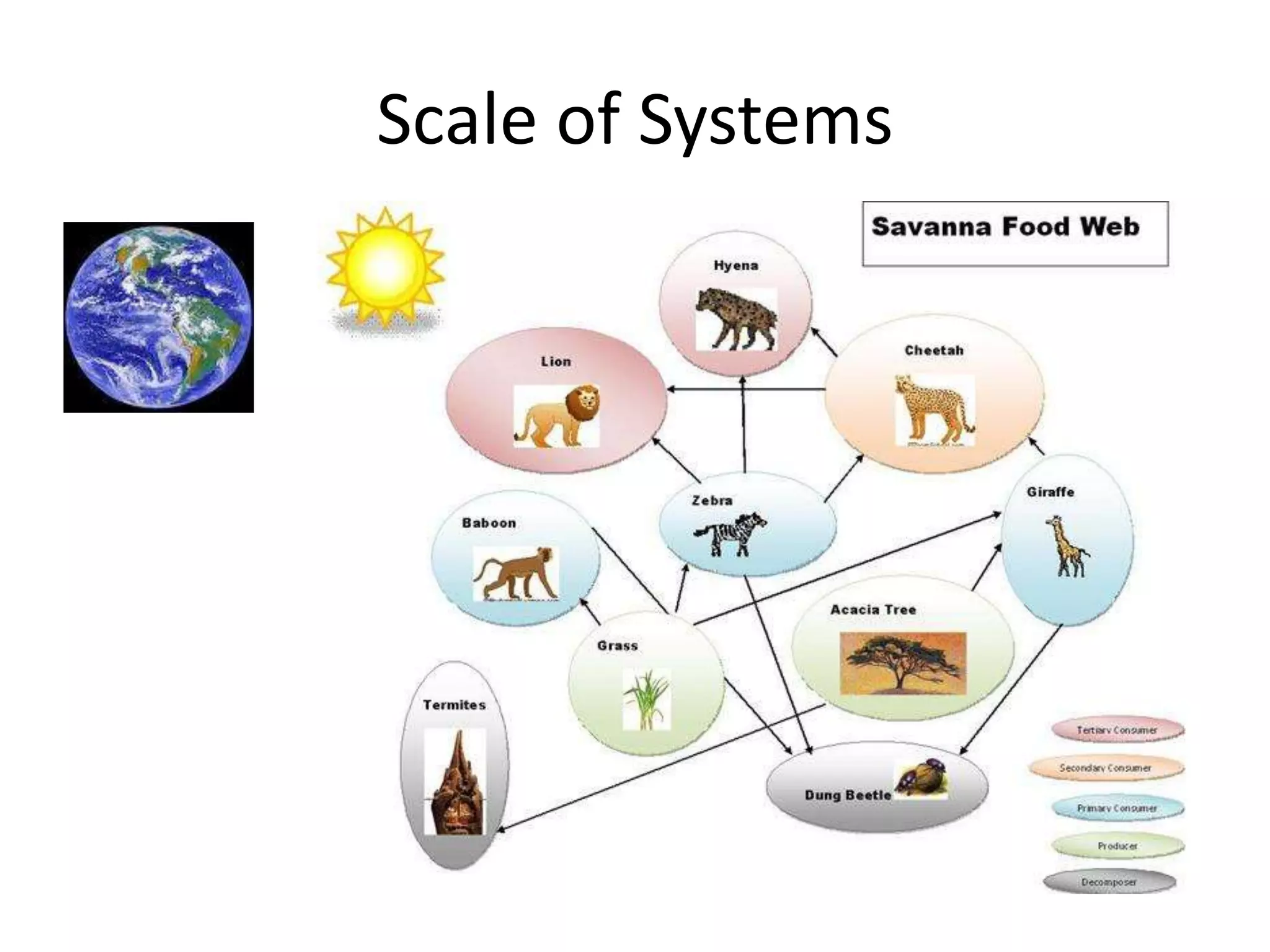
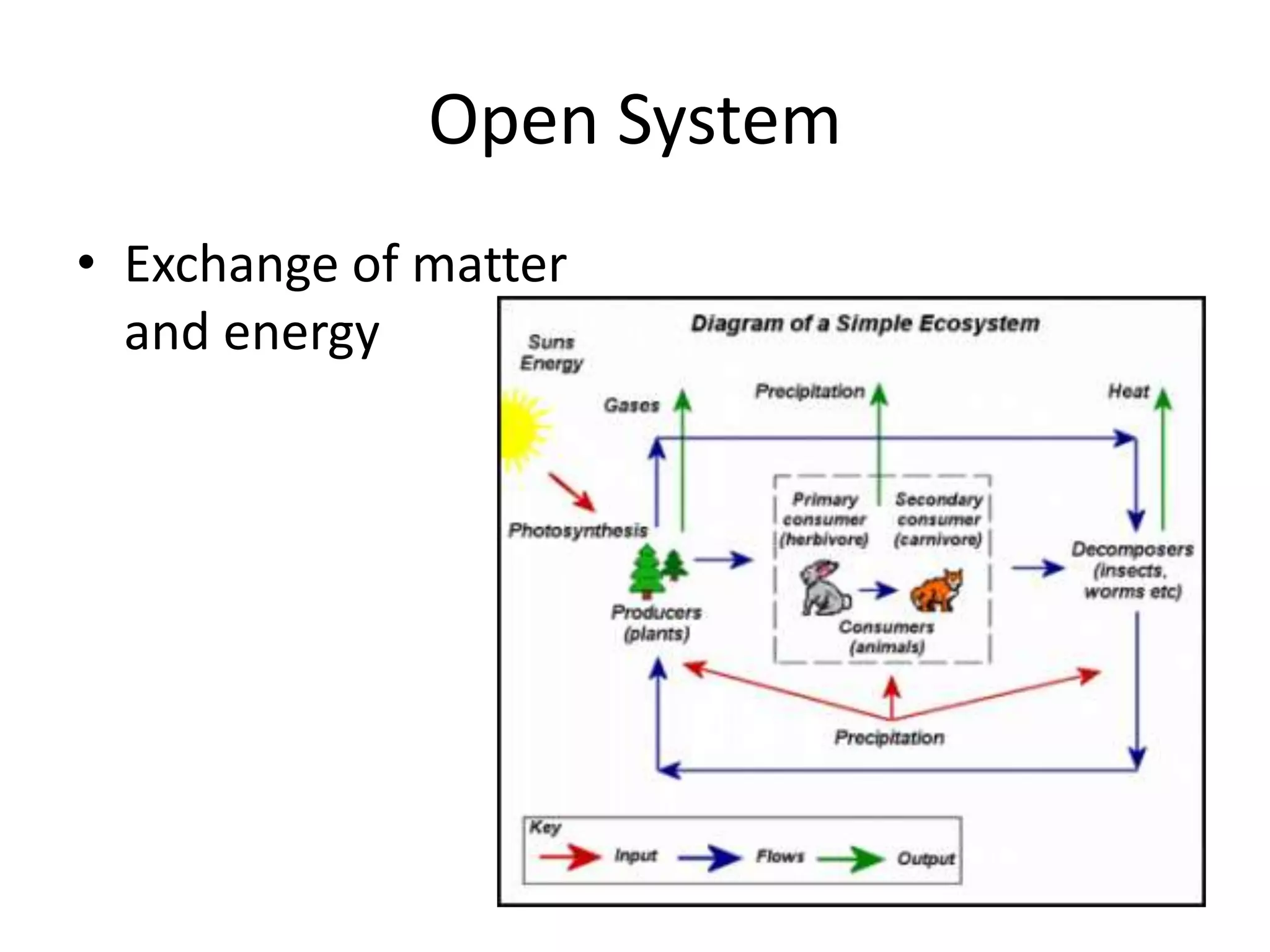
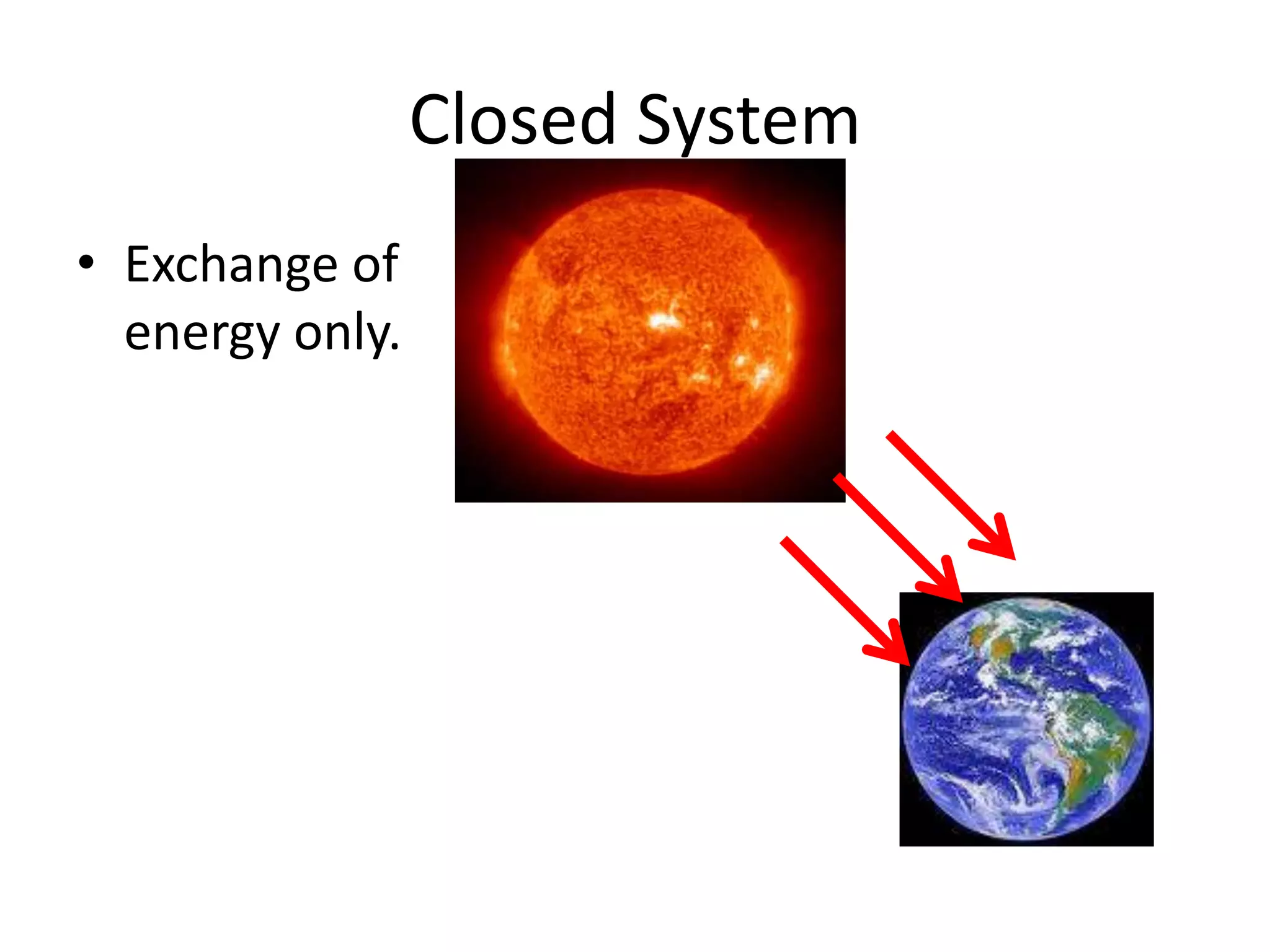
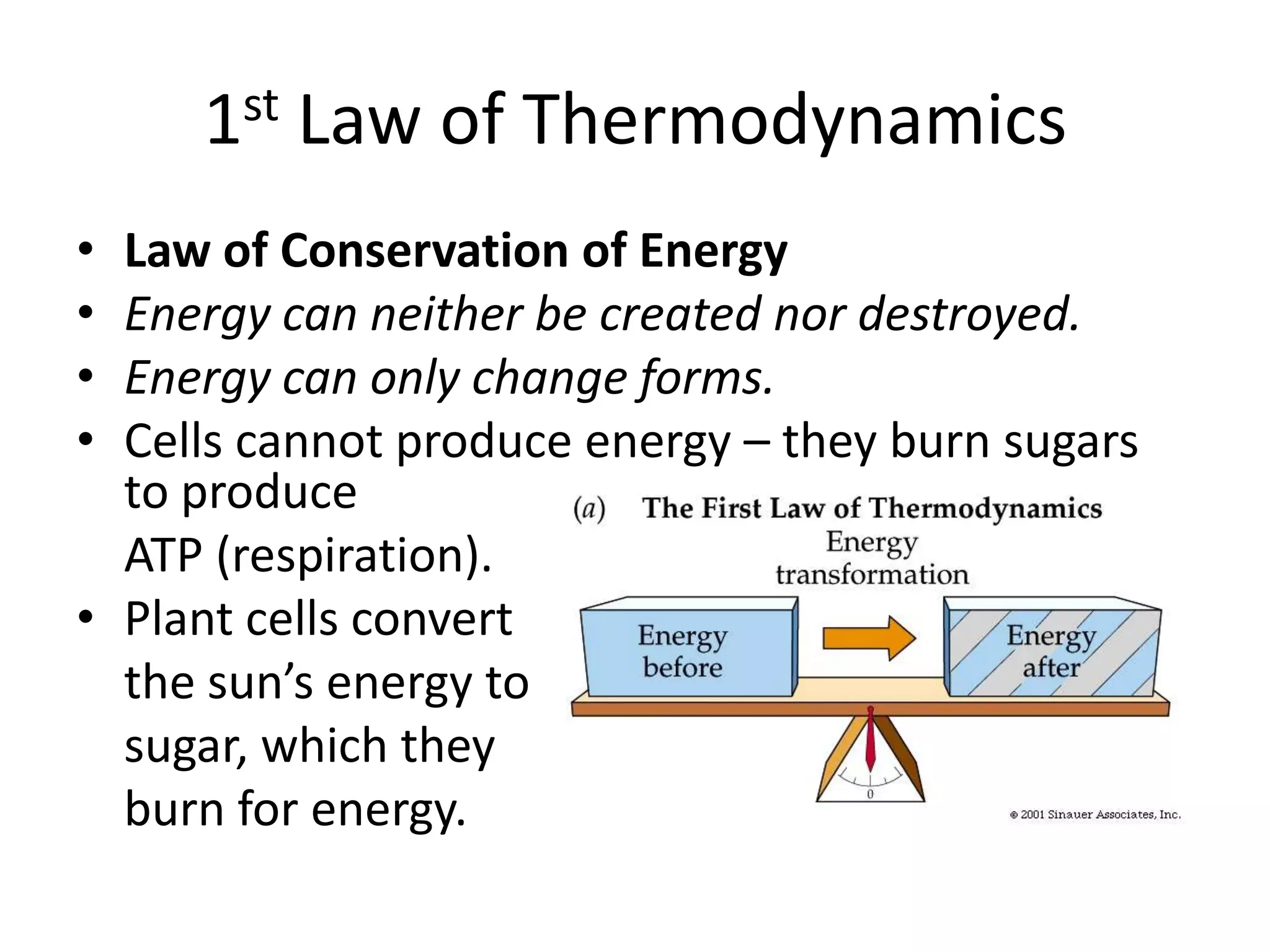
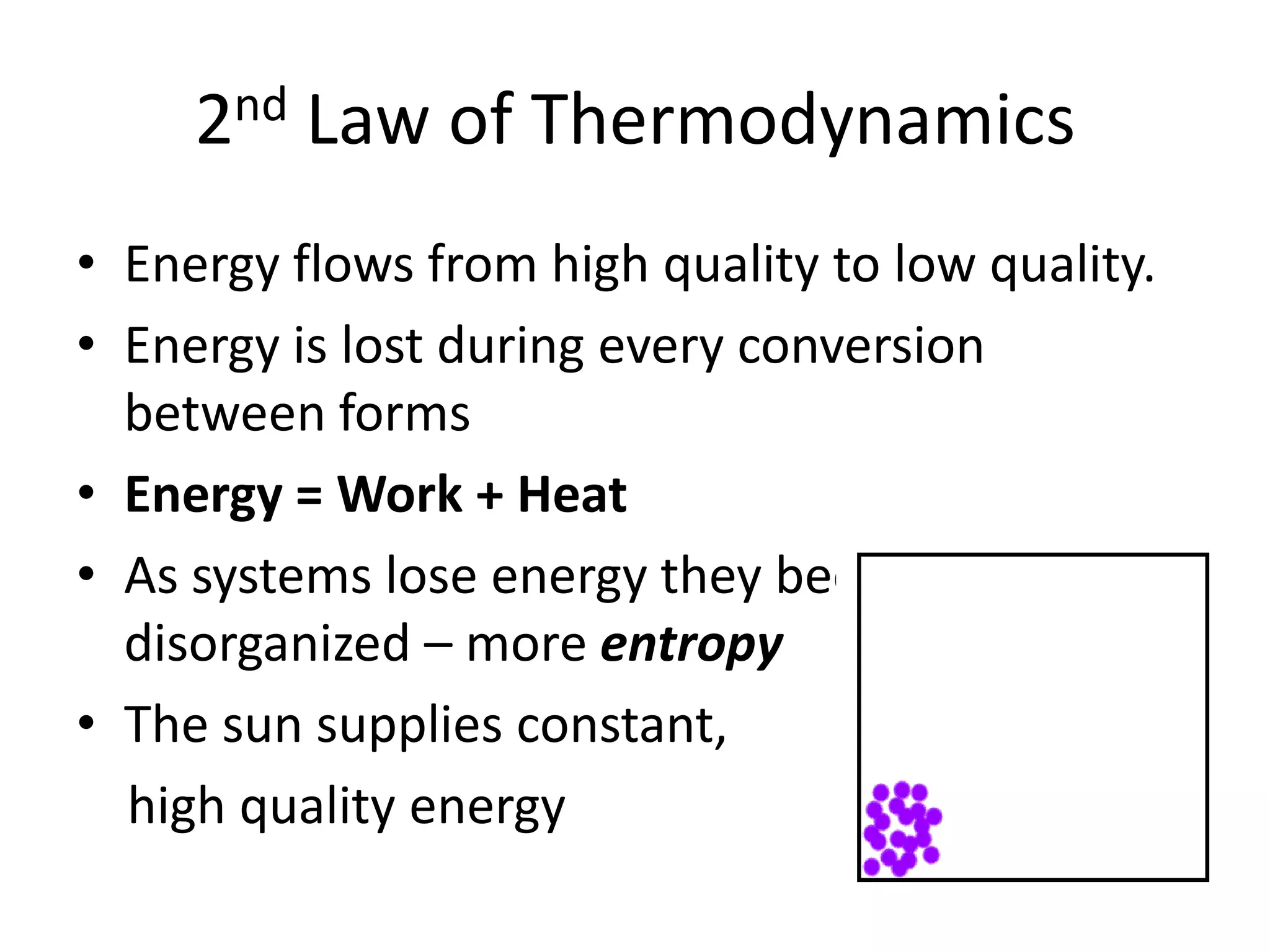
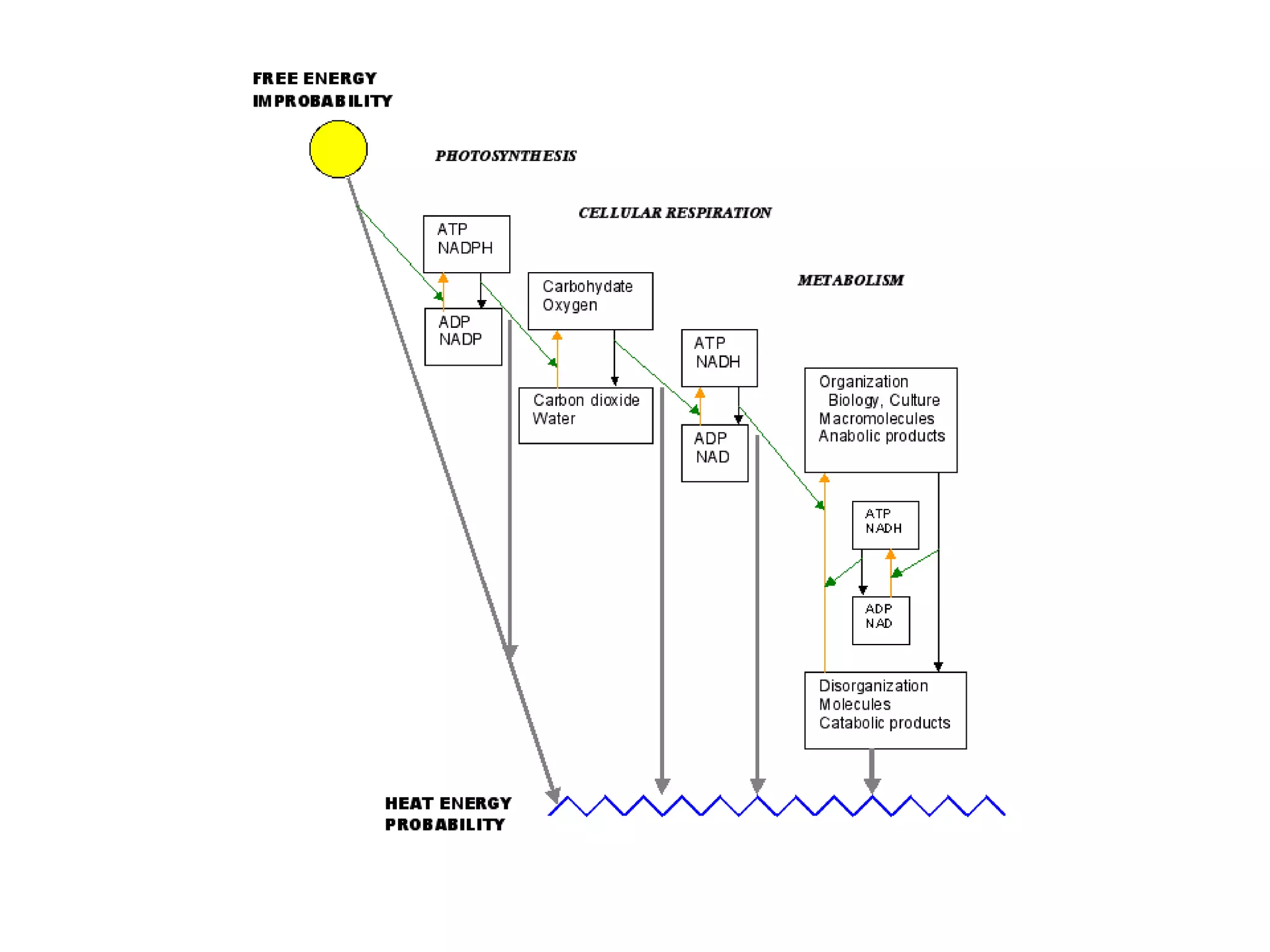
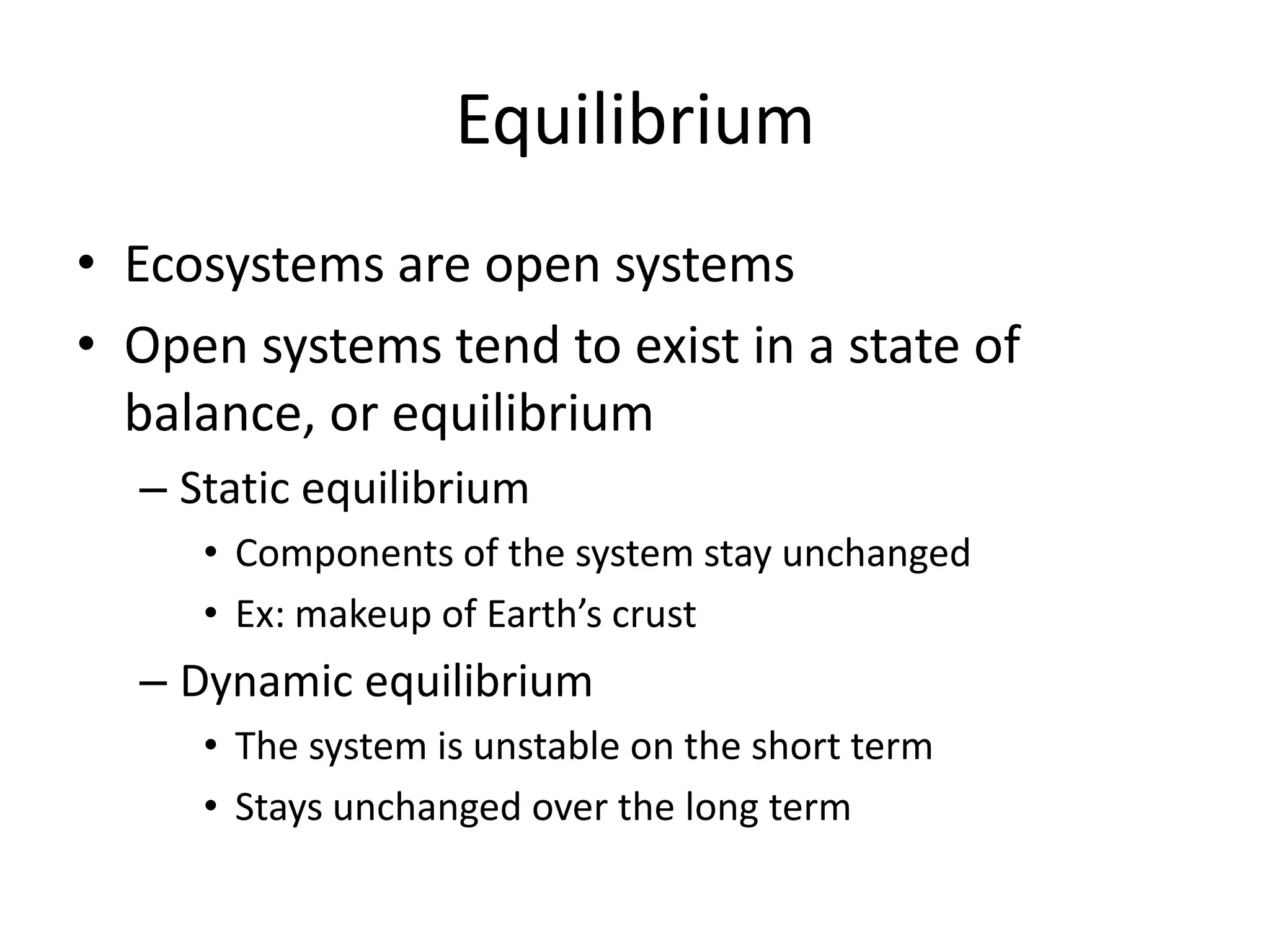
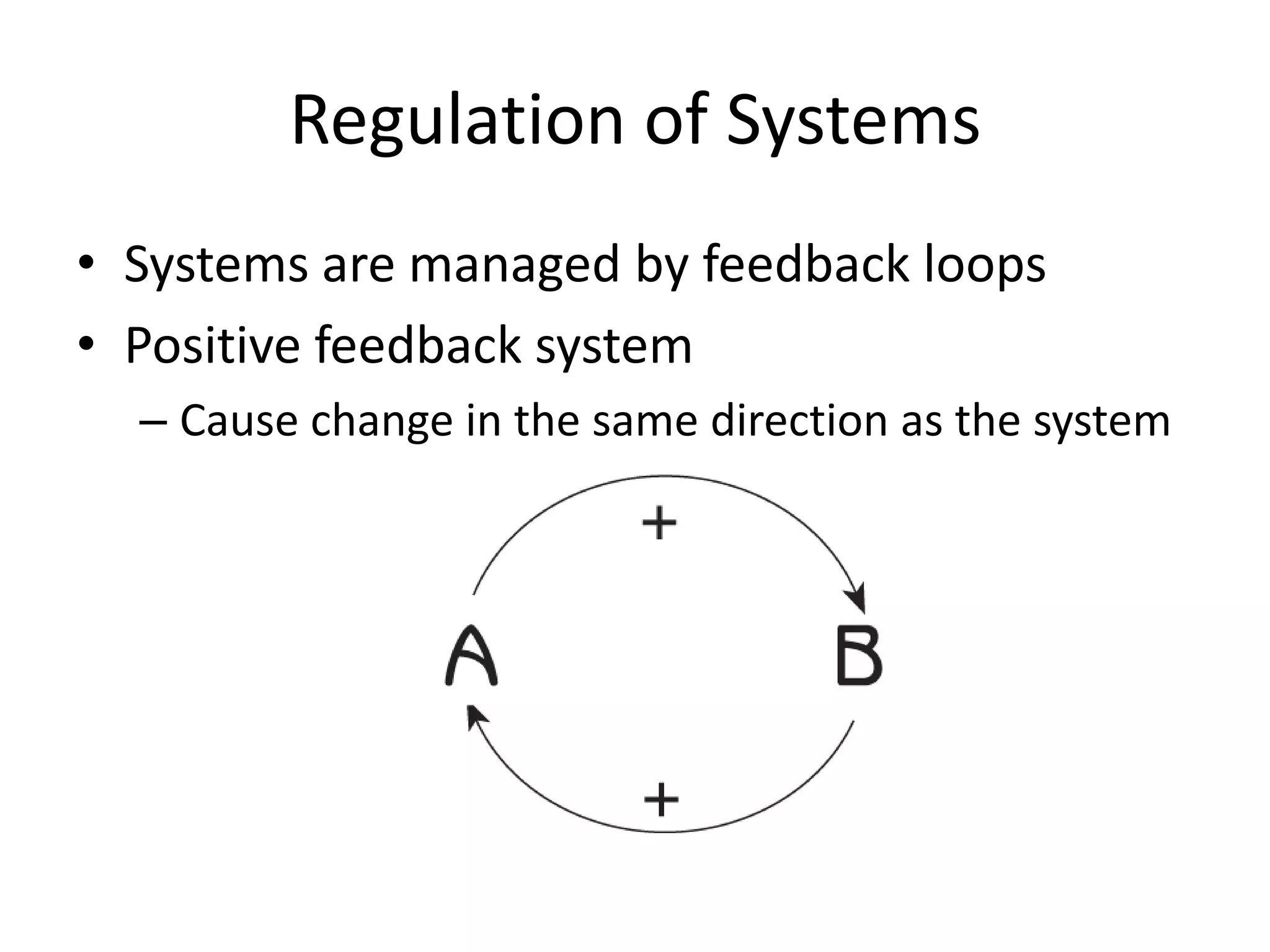
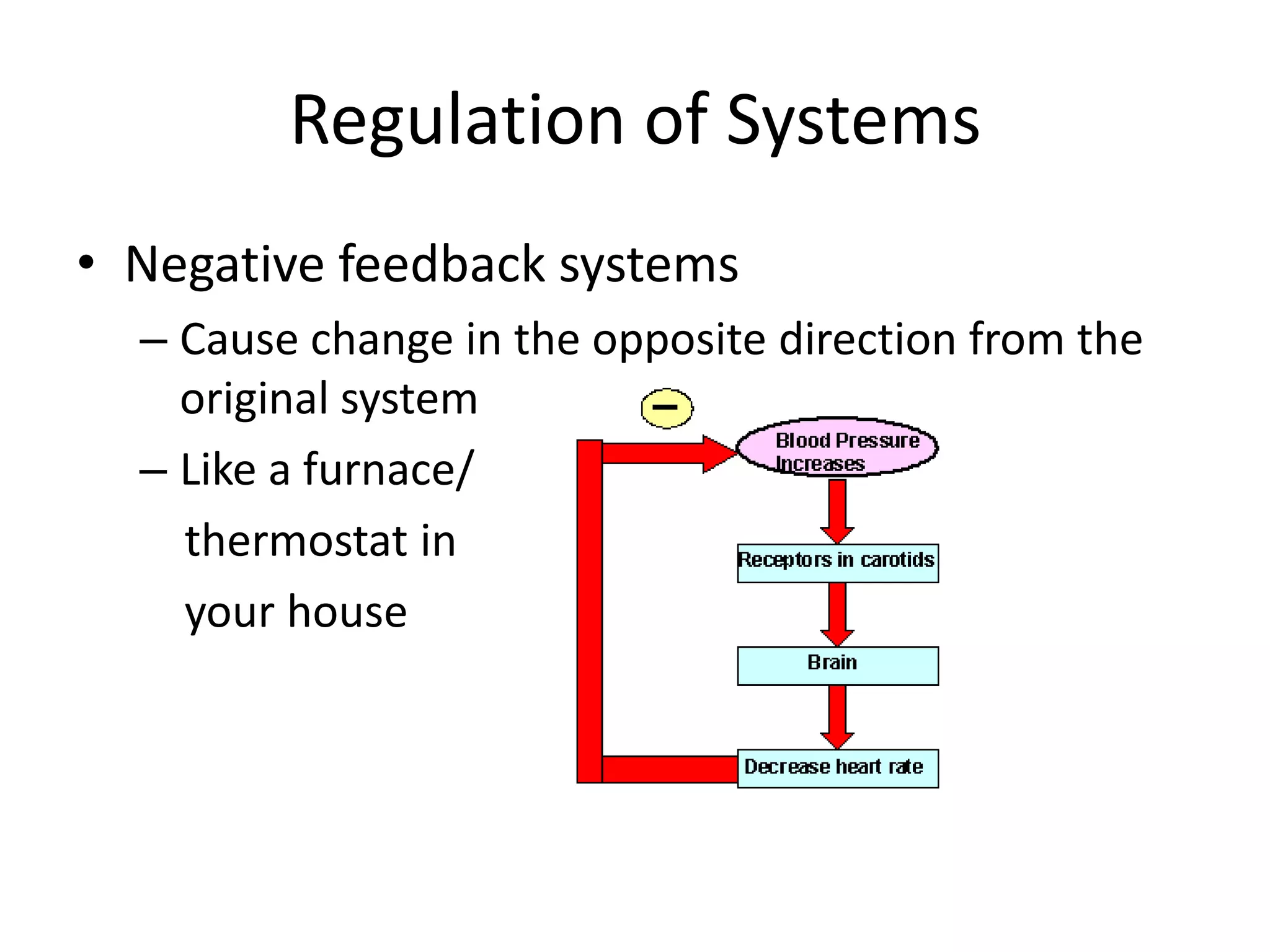
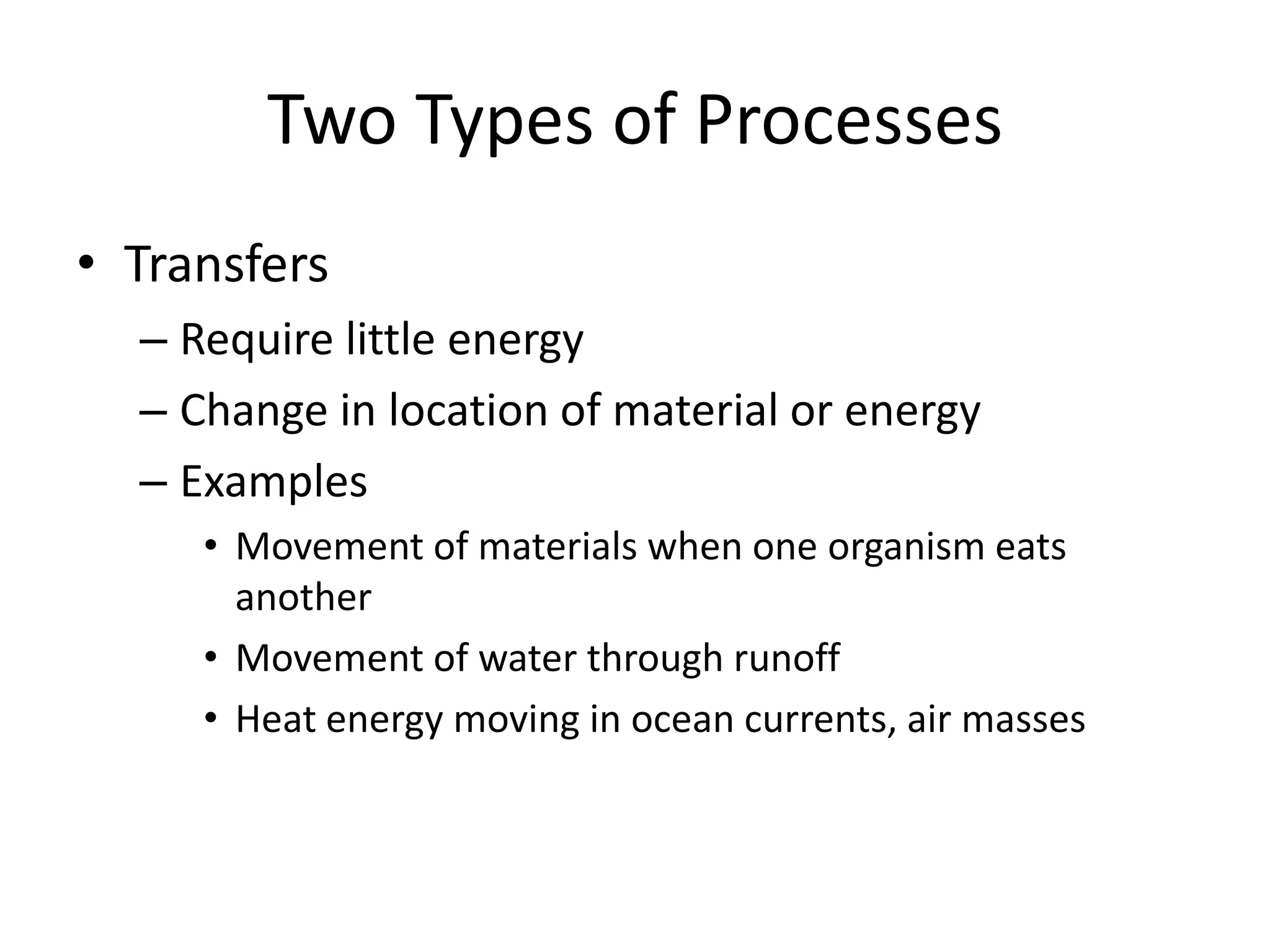
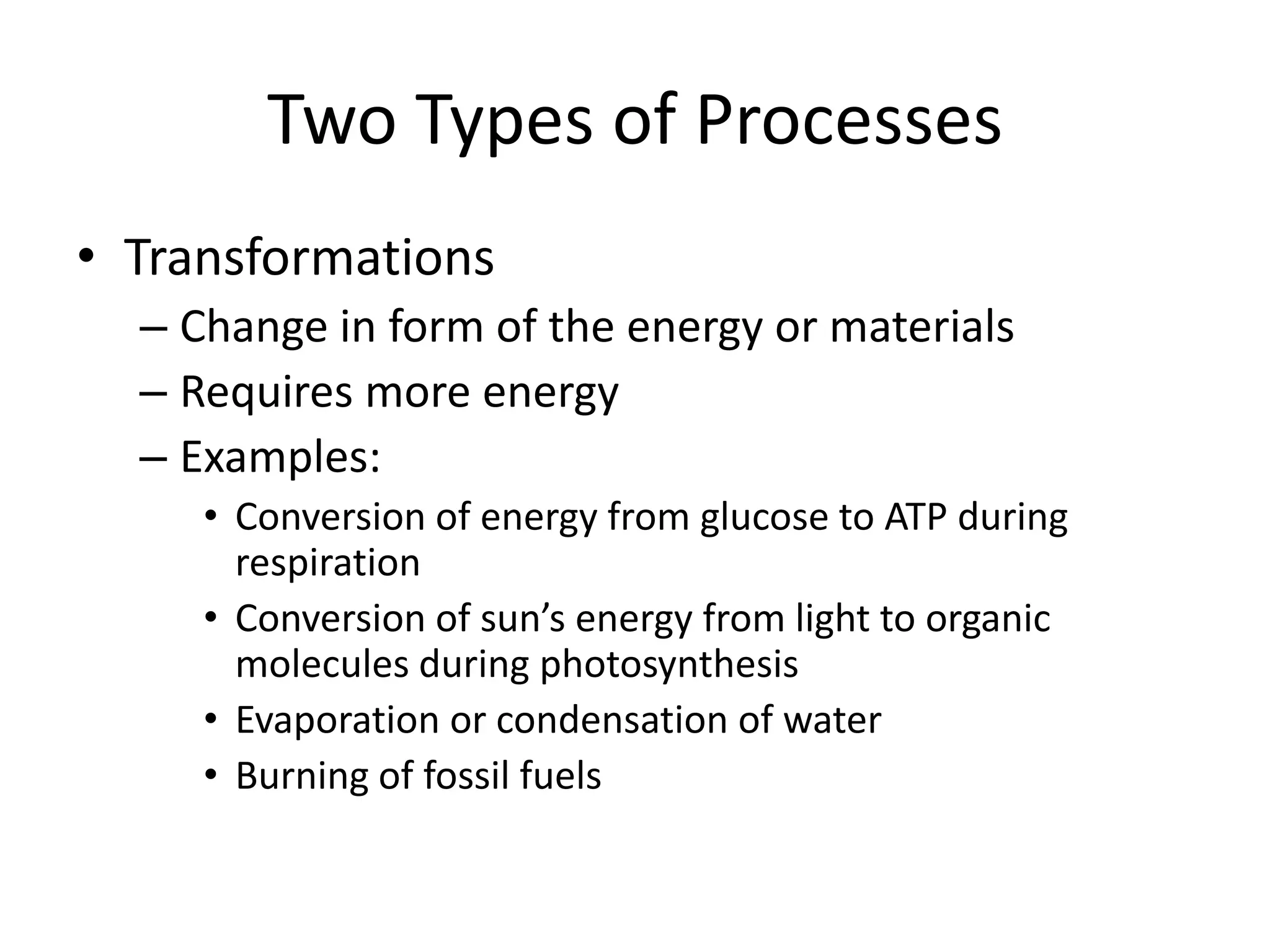
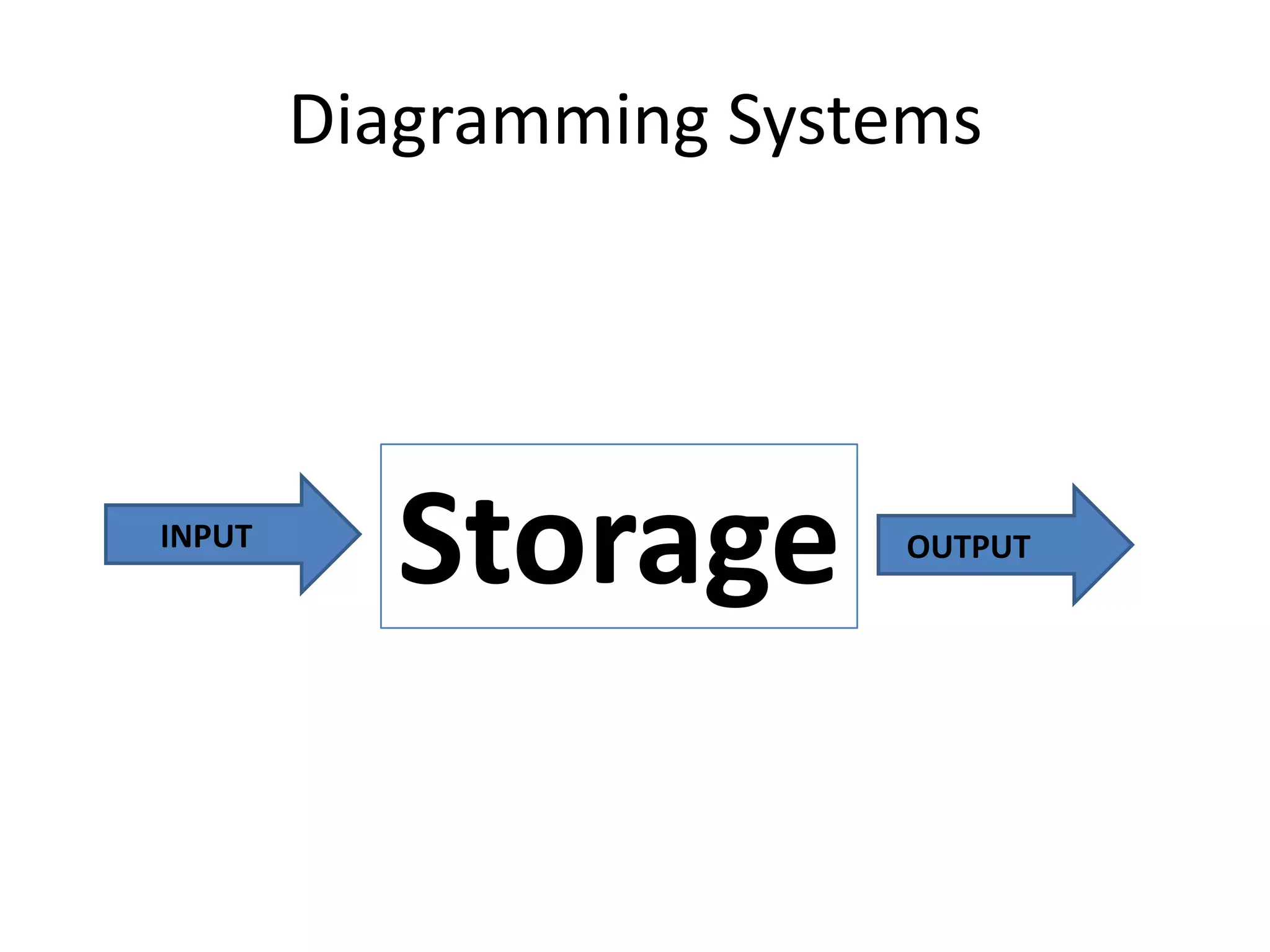
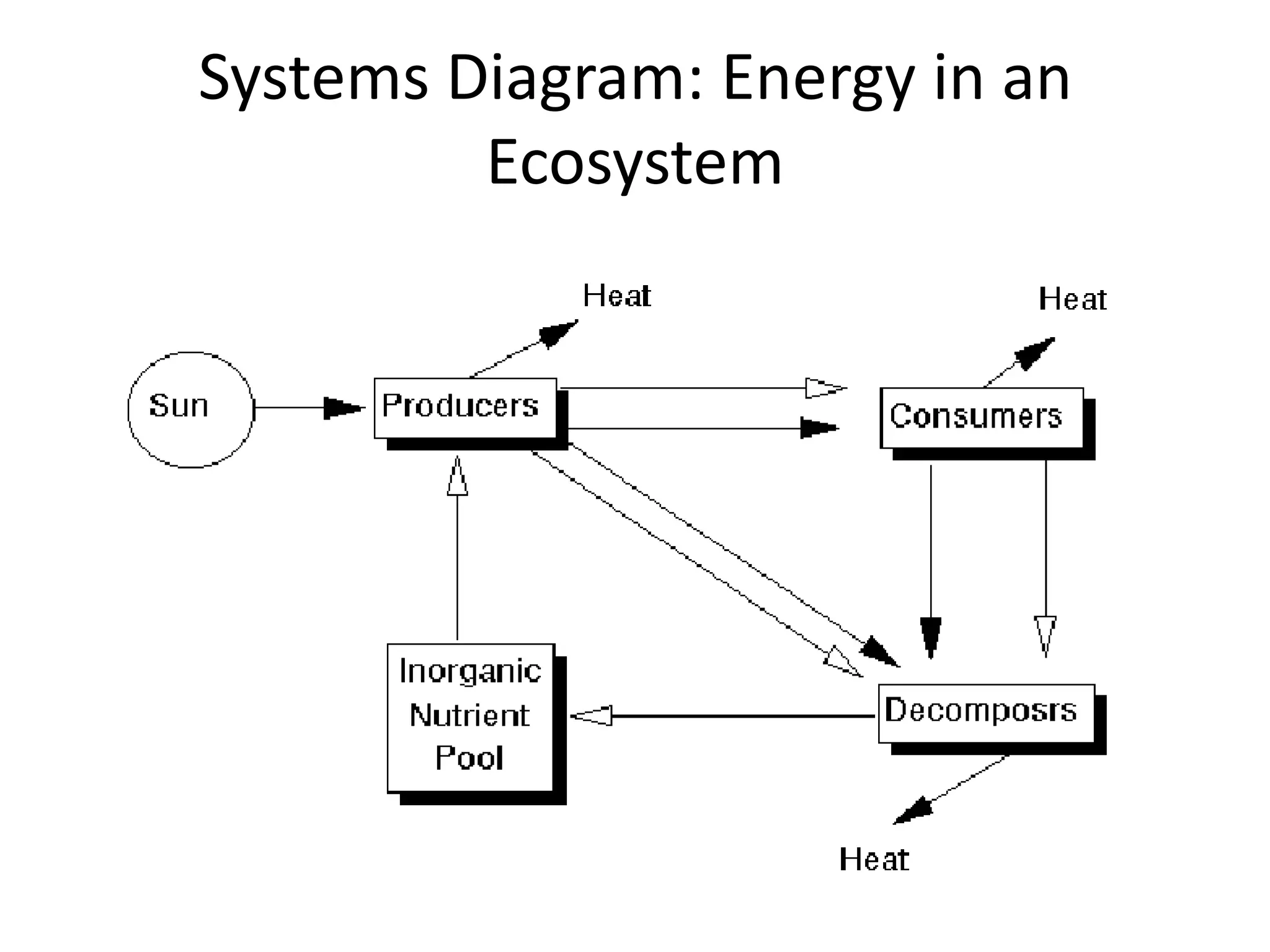
Systems consist of interacting elements that form a complex whole. Systems can be open, closed, or isolated depending on whether they exchange matter, energy, or both. Energy flows through systems and is lost during transformations from one form to another, as stated by the second law of thermodynamics. Ecosystems are open systems that maintain dynamic equilibrium through negative feedback loops, with energy driving materials through processes of transfer and transformation.