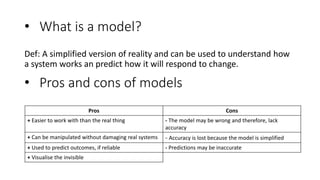
1. Models are simplified representations of reality that can be used to understand and predict how systems respond to change. While easier to work with than real systems, models may lack accuracy and predictions can be inaccurate.
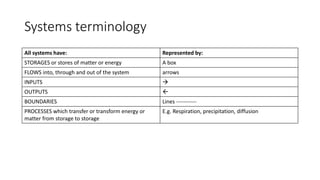
2. Systems are sets of interrelated parts that work together to form a complex whole. They can be living or non-living and exist on any scale. Systems are represented by storages, flows, inputs, outputs, boundaries, and processes that transfer matter and energy between storages.
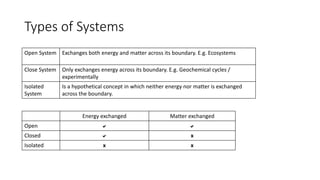
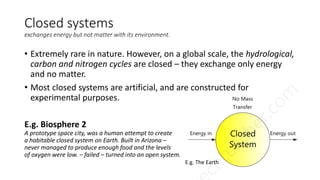
3. There are three types of systems: open systems exchange both matter and energy, closed systems exchange only energy, and isolated systems exchange neither matter nor energy. Most living systems are open while global biogeochemical cycles