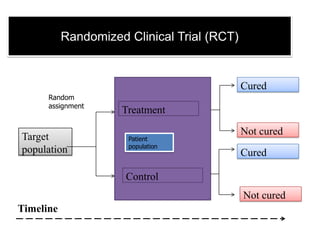
This document discusses survival analysis techniques. It begins with an overview of survival, censoring, and the need for survival analysis when not all patients have died or had the event of interest. It then describes the key techniques of life tables/actuarial analysis and the Kaplan-Meier method. Life tables involve constructing a hypothetical cohort and estimating survival at different ages based on mortality rates. The Kaplan-Meier method is commonly used to illustrate survival curves and gives partial credit to censored observations. A modified life table is also presented to analyze survival outcomes in different treatment groups.
































![Life table for sample of 13 patient treated with etoposide with cisplatin
Life Table Survival Variable: Progression-Free Survival
ni
wi
di
No. of pts (13) began the
study, so n1 is 13
Interval
No.
No.
No.
No. of
Start entering withdrawn exposed terminal
2 patients are
Time
Interval
du.referredto risk
events
to as
Interval
withdrawals (w1).
qi = di/[ni- pi = 1–qi si = pipi–1pi(wi/2)]
2…p1
Propn
Propn
terminating surviving
Cumul Propn
Surv at End
0.0
13.0
2.0
12.0
1.0
1 patient's disease
0.0833 progressed, referred
0.9167
0.9167
to as a terminal
event (d1)
3.0
10.0
4.0
8.0
1.0
0.1250
0.8750
0.8021
6.0
5.0
4.0
3.0
0.0
0.0000
1.0000
0.8021
9.0
1.0
1.0
0.5
0.0
0.0000
1.0000
0.8021
Source: Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, et al: Irinotecan plus cisplatin
compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002; 346: 85–](https://image.slidesharecdn.com/survivalanalysis-140131062719-phpapp02/85/Survival-analysis-34-320.jpg)

![Life table for sample of 13 patient treated with etoposide with cisplatin
Life Table Survival Variable: Progression-Free Survival
ni
wi
Interval
No.
No.
Start entering withdrawn
Time
Interval
du.
Interval
One-half of the number of patients
di
qi = di/[niwithdrawing is subtracted from the pi = 1–qi
(wi/2)]
number beginning the interval, so the
EXPOSED TO RISK during the
period, 13 – No. of2), or 12 in first
(½
No.
Propn
Propn
interval. terminal terminating surviving
exposed
to risk
si = pipi–1pi2…p1
Cumul Propn
Surv at End
events
0.0
13.0
2.0
12.0
1.0
0.0833
0.9167
0.9167
3.0
10.0
4.0
8.0
1.0
0.1250
0.8750
0.8021
6.0
5.0
4.0
3.0
0.0
0.0000
1.0000
0.8021
9.0
1.0
1.0
0.5
0.0
0.0000
1.0000
0.8021
Source: Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, et al: Irinotecan plus cisplatin
compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002; 346: 85–](https://image.slidesharecdn.com/survivalanalysis-140131062719-phpapp02/85/Survival-analysis-36-320.jpg)
![Life table for sample of 13 patient treated with etoposide with cisplatin
Life Table Survival Variable: Progression-Free Survival
ni
wi
Interval
No.
No.
Start entering withdrawn
Time
Interval
du.
Interval
di
No.
exposed
to risk
qi = di/[ni- pi = 1–qi si = pipi–1pi(wi/2)]
2…p1
The of
No. proportion terminating (q1
Propn
Propn
= d1/[n1-(w1/2]) is 1/12 =
terminal terminating surviving
0.0833.
events
Cumul Propn
Surv at End
0.0
13.0
2.0
12.0
1.0
0.0833
0.9167
0.9167
3.0
10.0
4.0
8.0
1.0
0.1250
0.8750
0.8021
6.0
5.0
4.0
3.0
0.0
0.0000
1.0000
0.8021
9.0
1.0
1.0
0.5
0.0
0.0000
1.0000
0.8021
Source: Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, et al: Irinotecan plus cisplatin
compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002; 346: 85–](https://image.slidesharecdn.com/survivalanalysis-140131062719-phpapp02/85/Survival-analysis-37-320.jpg)
![Life table for sample of 13 patient treated with etoposide with cisplatin
Life Table Survival Variable: Progression-Free Survival
ni
wi
Interval
No.
No.
Start entering withdrawn
Time
Interval
du.
Interval
di
No.
exposed
to risk
qi = di/[ni- pi = 1–qi si = pipi–1pi(wi/2)]
2…p1
No. of
Propn
Propn
Cumul Propn
terminal proportion surviving (p1 = 1-q1) is 1End
Surv at –
The terminating surviving
events
0.0833 = 0.9167
0.0
13.0
2.0
12.0
1.0
0.0833
0.9167
3.0
10.0
4.0
8.0
1.0
0.1250
0.8750 we are still in
0.8021
because
6.0
5.0
4.0
3.0
0.0
0.0000
9.0
1.0
1.0
0.5
0.0
0.0000
0.9167
the first period, the
cumulative survival is
1.0000
0.8021
0.9167
1.0000
0.8021
Source: Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, et al: Irinotecan plus cisplatin
compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002; 346: 85–](https://image.slidesharecdn.com/survivalanalysis-140131062719-phpapp02/85/Survival-analysis-38-320.jpg)
![Life table for sample of 13 patient treated with etoposide with cisplatin
Life Table Survival Variable: Progression-Free Survival
ni
wi
di
Interval
No.
No.
No.
No. of
the
Start At entering withdrawn exposed terminal
Time beginning of
Interval
du.
to risk
events
the second
four
Interval patients
withdraw w2 = 4
interval, only
0.0 10 patients
13.0
2.0
12.0
1.0
remain.n2=10
3.0
10.0
4.0
8.0
1.0
6.0
5.0
4.0
3.0
0.0
9.0
1.0
1.0
0.5
0.0
qi = di/[ni- pi = 1–qi si = pipi–1pi(wi/2)]
2…p1
Propn
Propn
terminating surviving
0.0833
0.9167
one's
0.1250
0.8750
disease
progressed,
so d2 = 1
Cumul Propn
Surv at End
0.9167
0.8021
0.0000
1.0000
0.8021
0.0000
1.0000
0.8021
Source: Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, et al: Irinotecan plus cisplatin
compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002; 346: 85–](https://image.slidesharecdn.com/survivalanalysis-140131062719-phpapp02/85/Survival-analysis-39-320.jpg)
![Life table for sample of 13 patient treated with etoposide with cisplatin
Life Table Survival Variable: Progression-Free Survival
ni
wi
di
Interval
No.
No.
No.
No. of
Start entering withdrawn exposed terminal
Time
Interval
du.
to risk
events
Interval
the proportion terminating (q2
nd
0.0
13.0 = d2/[n2-(w2/2]) during 2
2.0
12.0
1.0
interval is 1/[10 – (4/2)] =
1/8, or 0.1250.
qi = di/[ni- pi = 1–qi si = pipi–1pi(wi/2)]
2…p1
Propn
Propn proportion
the Cumul Propn
terminating surviving no
with Surv at End
progression is
1 – 0.1250, or
0.8750 0.9167
0.0833
0.9167
3.0
10.0
4.0
8.0
1.0
0.1250
0.8750
0.8021
6.0
5.0
4.0
3.0
0.0
0.0000
1.0000
0.8021
9.0
1.0
1.0
0.5
0.0
0.0000
1.0000
0.8021
Source: Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, et al: Irinotecan plus cisplatin
compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002; 346: 85–](https://image.slidesharecdn.com/survivalanalysis-140131062719-phpapp02/85/Survival-analysis-40-320.jpg)
![Life table for sample of 13 patient treated with etoposide with cisplatin
Life Table Survival Variable: Progression-Free Survival
ni
wi
Interval
No.
No.
Start entering withdrawn
Time
Interval
du.
Interval
0.0
3.0
13.0
10.0
2.0
di
No.
exposed
to risk
No. of
terminal
events
qi = di/[ni- pi = 1–qi si = pipi–1pi(wi/2)]
2…p1
Propn
Propn
terminating surviving
the
12.0 cumulative proportion of surv
1.0
0.0833
0.9167
4.0
8.0
6.0 from probability theory:
5.0
4.0
Rule
3.0
=p1*p2= 0.0.9167 × 0.8750=
0.8021
Cumul Propn
Surv at End
0.9167
1.0
0.1250
0.8750
0.8021
0.0
0.0000
1.0000
0.8021
0.0
0.0000
1.0000
0.8021
P(A&B)=P(A)*P(B) if A and B independent
9.0
1.0
1.0
0.5
Source: Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, et al: Irinotecan plus cisplatin
compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002; 346: 85–9](https://image.slidesharecdn.com/survivalanalysis-140131062719-phpapp02/85/Survival-analysis-41-320.jpg)



































