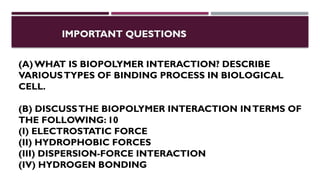
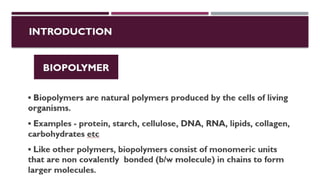
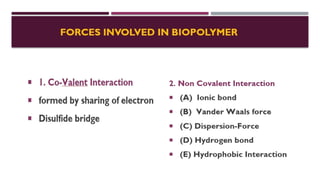
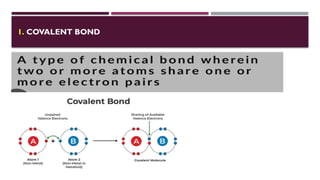
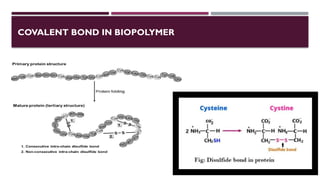
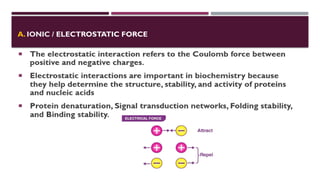
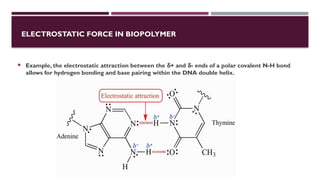
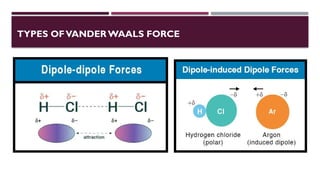
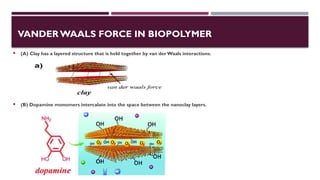
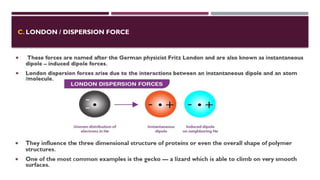
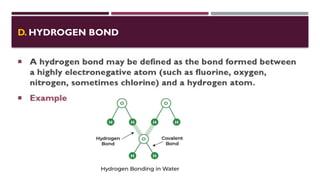
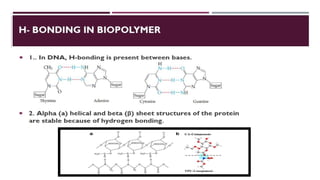
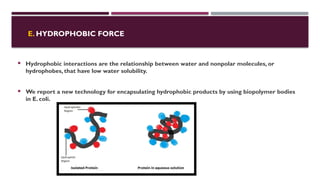
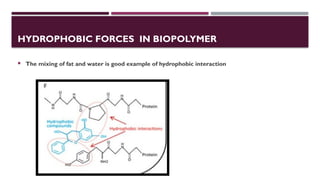
The document discusses biopolymer interactions, detailing various binding processes in biological cells, including covalent and non-covalent bonds. Key forces such as electrostatic, hydrophobic, van der Waals, and hydrogen bonding are examined, illustrating their importance in structures like DNA and proteins. Additionally, it highlights a new technology for encapsulating hydrophobic products using biopolymer bodies in E. coli.