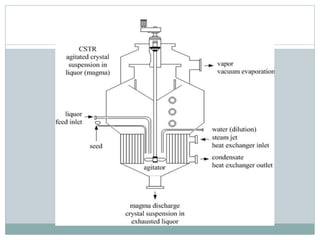
The document details the processes of sugar crystallisation, emphasizing nucleation and crystal growth as the two major steps. It describes how a vacuum pan is used to heat, evaporate, and create sugar crystals through controlled seeding and concentration of the juice. Additionally, it outlines the centrifugation process for separating sugar crystals from syrup, resulting in the production of white sugar and molasses.