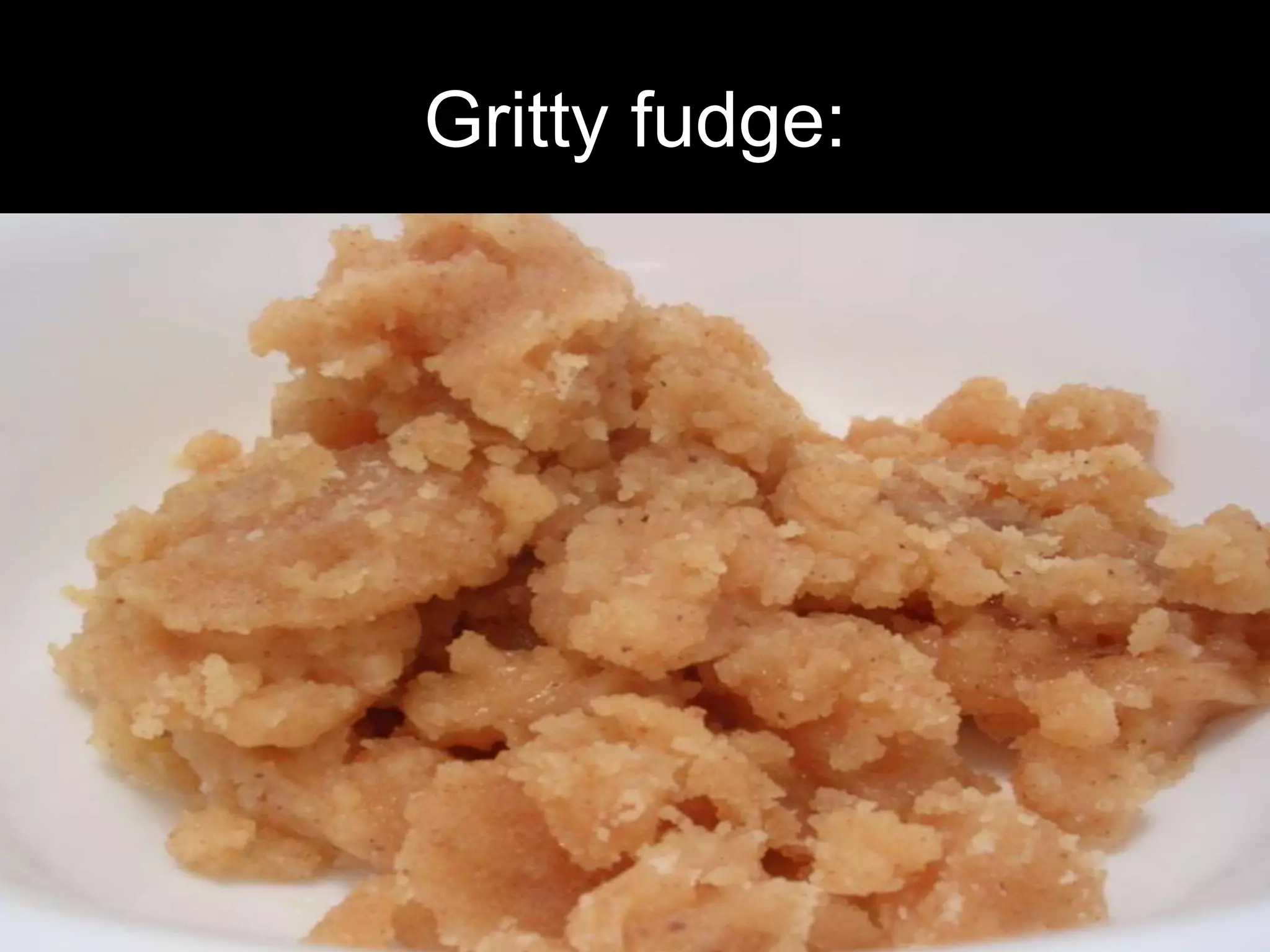
Fudge is a soft candy made from sugar, butter, and milk or cream, traditionally produced through a specific cooking process that controls sugar crystallization for texture. The history of fudge dates back about 100 years, with its origin linked to an accidental creation while attempting to make another candy type. Successful fudge relies on a balance of crystallization influenced by ingredients like glucose, fructose, and butter, as well as cooking conditions to achieve a creamy consistency.