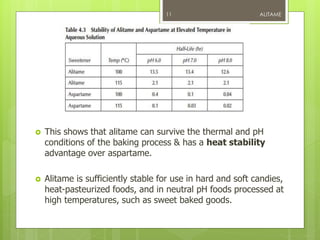
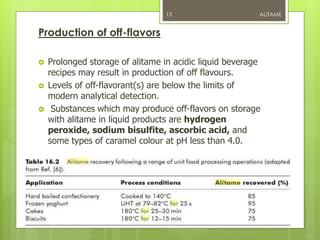
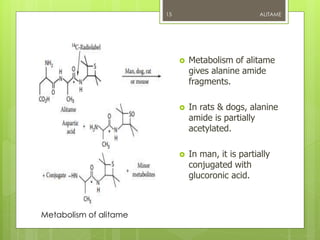
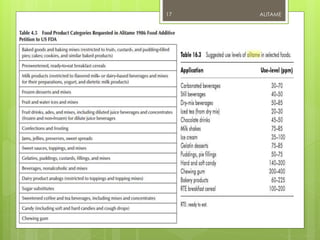
Alitame is an artificial sweetener that is 2000 times sweeter than sucrose. It is a dipeptide composed of L-aspartic acid and D-alanine, attached to a thietanyl group. Alitame is heat stable, non-caloric, and does not promote tooth decay. It is used in foods and beverages as a sugar substitute. While considered safe, some studies have shown alitame can affect gut bacteria and impair glucose metabolism. Alitame received regulatory approval in several countries in the 1990s but was later withdrawn in the US.


![STRUCTURE
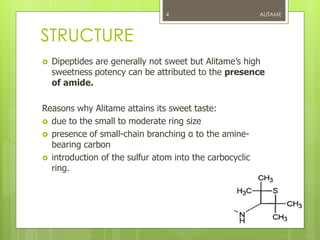
Alitame is a dipeptide of L-Aspartic acid & D-Alanine. Attached
to alanine is a amine (2,2,4,4 tetramethylthietanyl amine).
It was developed by Pfizer in the early 1980s and is currently
marketed in some countries under the brand name Aclame.
It was synthesized after the accidental discovery of Aspartame.
ALITAME3
IUPAC Name:
(3S)-3-amino-4-[ [(1R)-
1-methyl-2-oxo-2-
[(2,2,4,4-tetramethyl-3-
thietanyl)amino]ethyl]a
mino]-4-oxobutanoic
acid
L – Aspartic D- Alanine Amide](https://image.slidesharecdn.com/alitame-150718154713-lva1-app6892/85/Alitame-3-320.jpg)
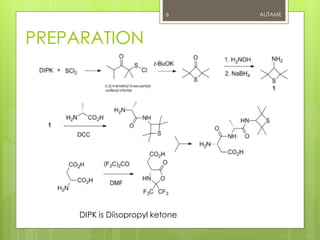
![PREPARATION
Alitame is prepared by a multistep synthesis involving
the reaction between two intermediates, (S)-[2,5-
dioxo-(4-thiazolidine)] acetic acid and (R)-2-amino-N-
(2,2,4,4-tetramethyl-3-thietanyl) propanamide.
The final product is isolated and purified through
crystallization of an alitame-4-methylbenzenesulfonic
acid molecules, followed by additional purification
steps, and finally recrystallization from water.
ALITAME5](https://image.slidesharecdn.com/alitame-150718154713-lva1-app6892/85/Alitame-5-320.jpg)