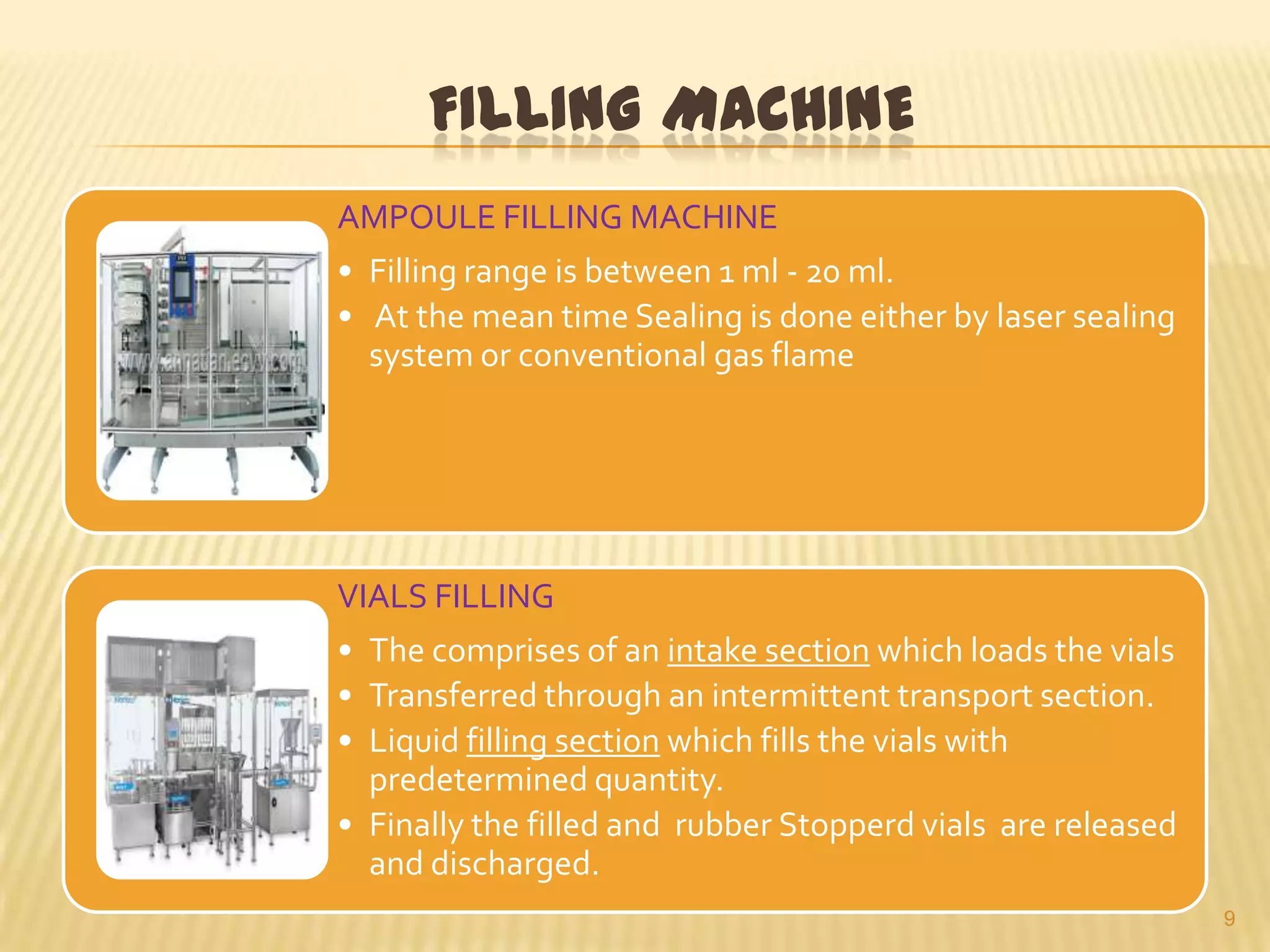
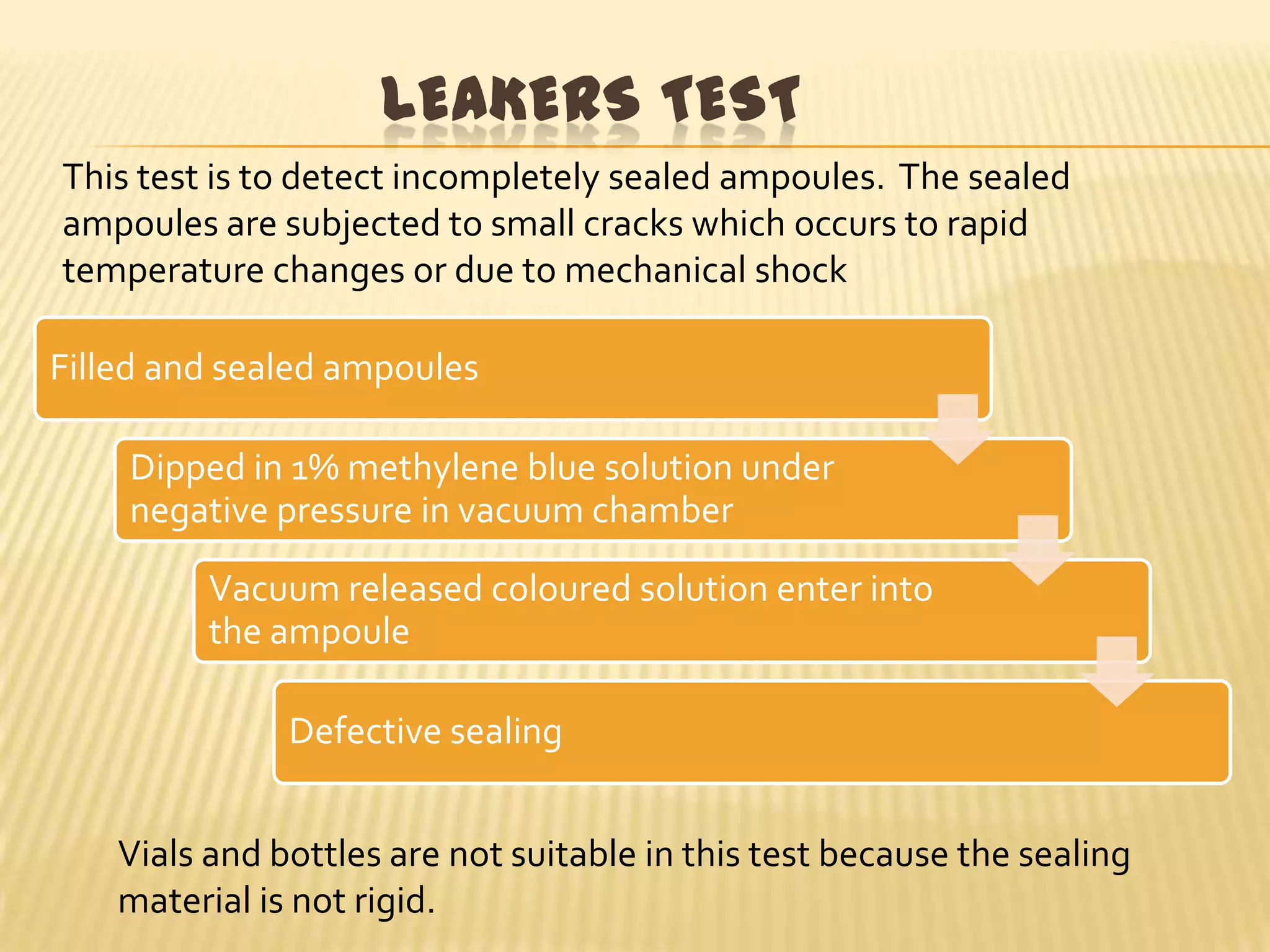
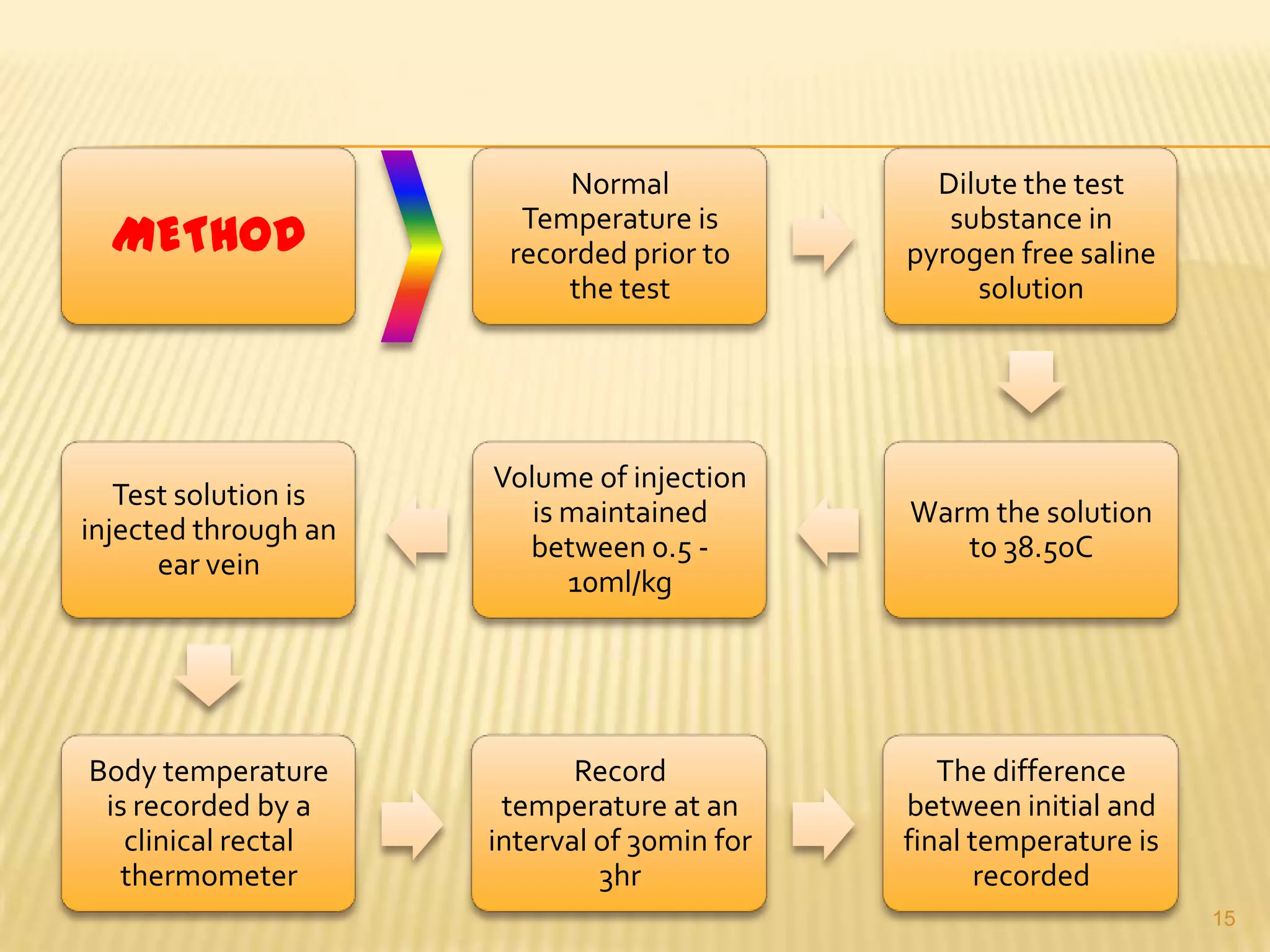
This document summarizes equipment and quality control procedures for the large-scale manufacture of parenteral products. It describes storage, washing, drying, filling and sealing equipment needed for manufacturing areas and aseptic rooms. Quality control includes leak testing, clarity testing, pyrogen testing using rabbit tests or LAL assays, and sterility testing to check for microorganisms. HEPA filters and laminar flow work stations are used to maintain sterile conditions.