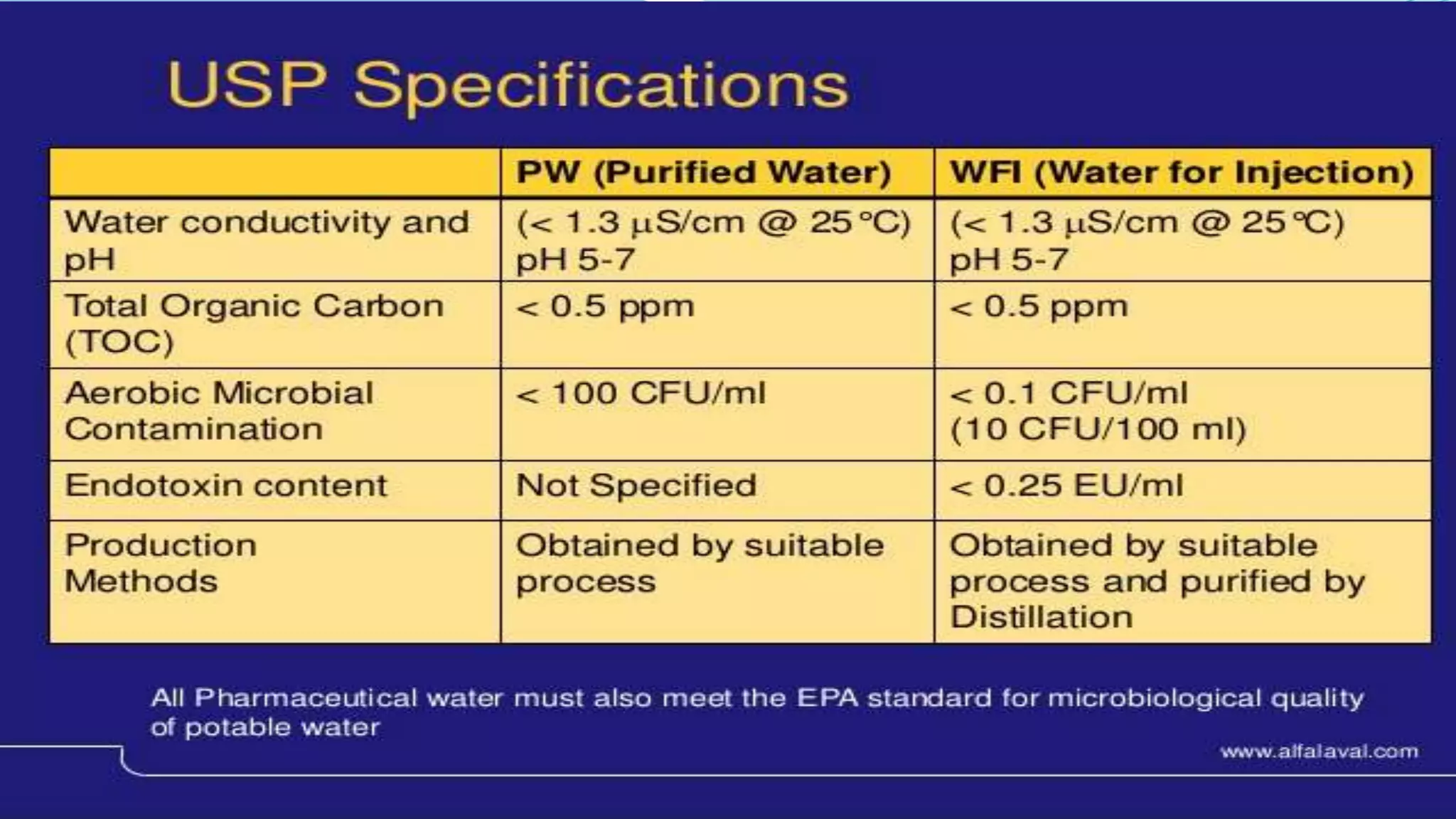
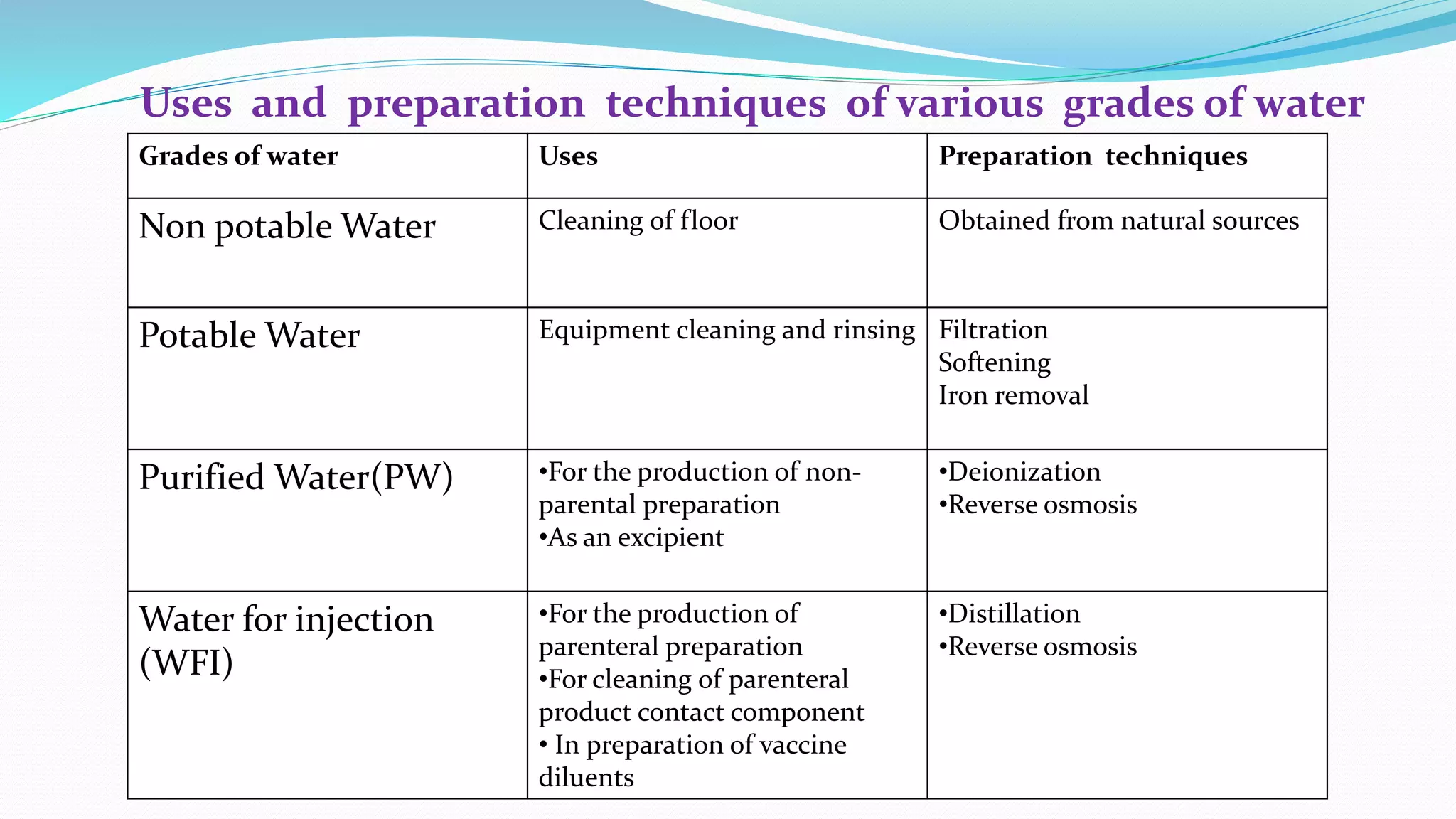
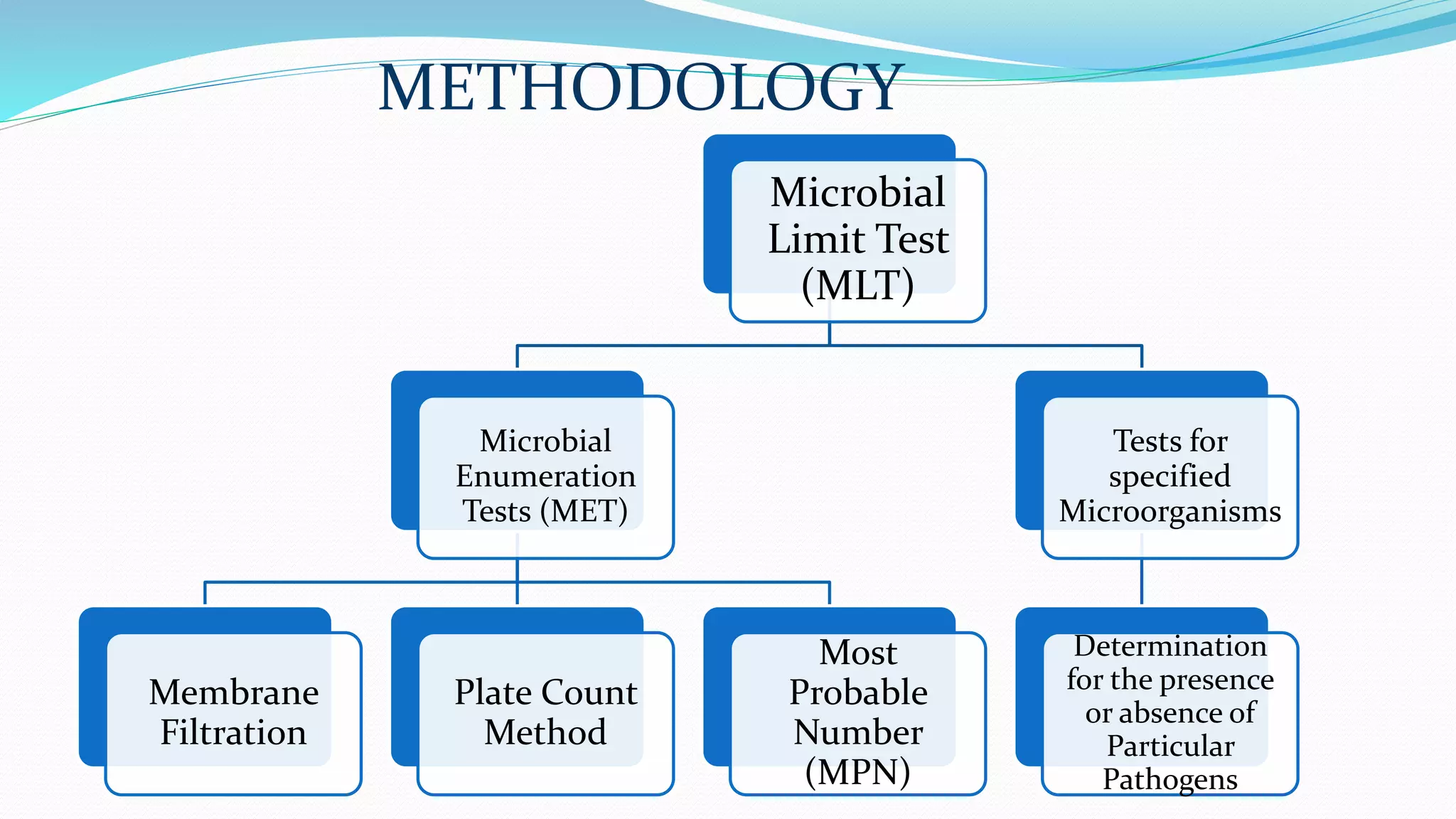
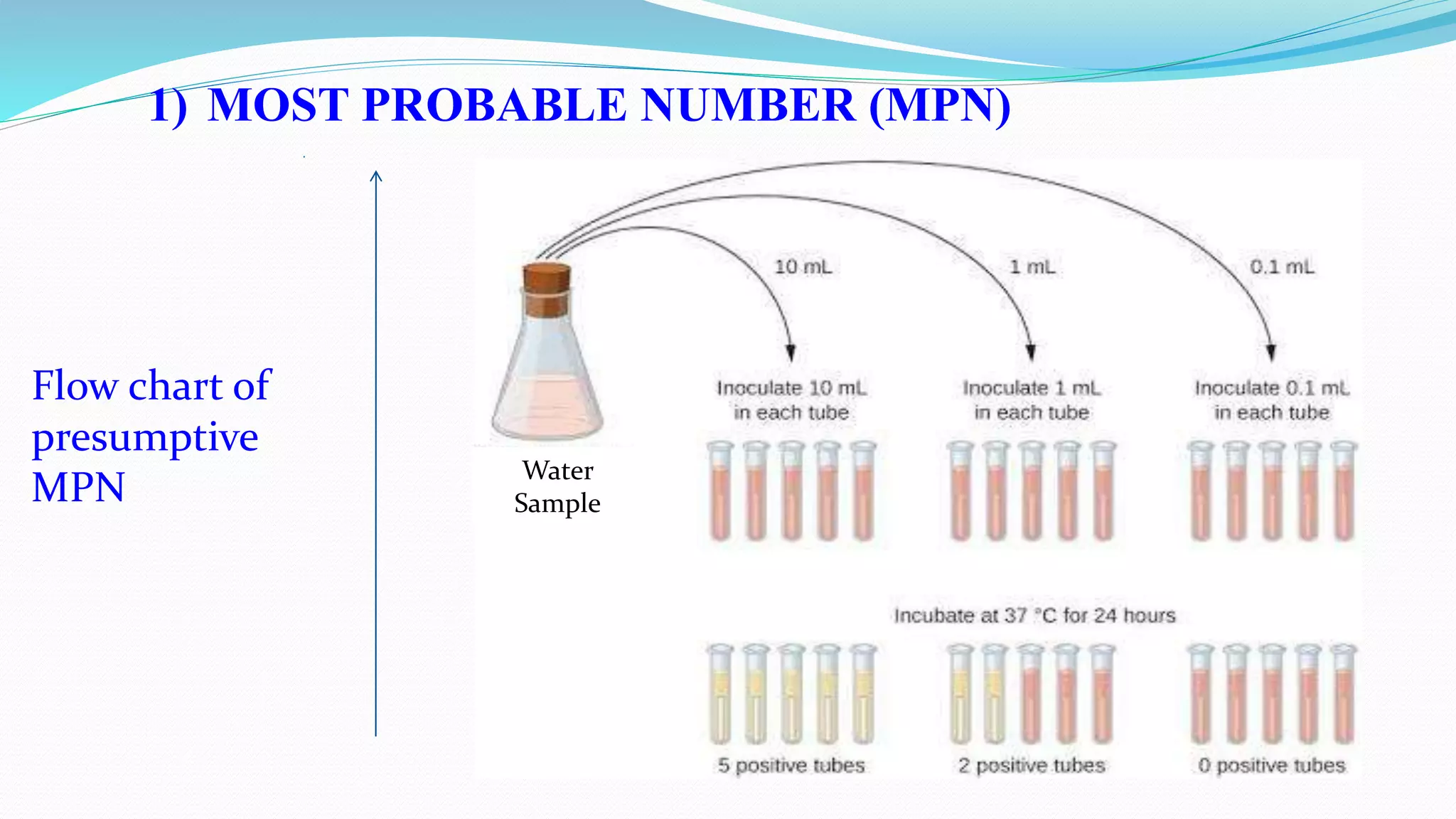
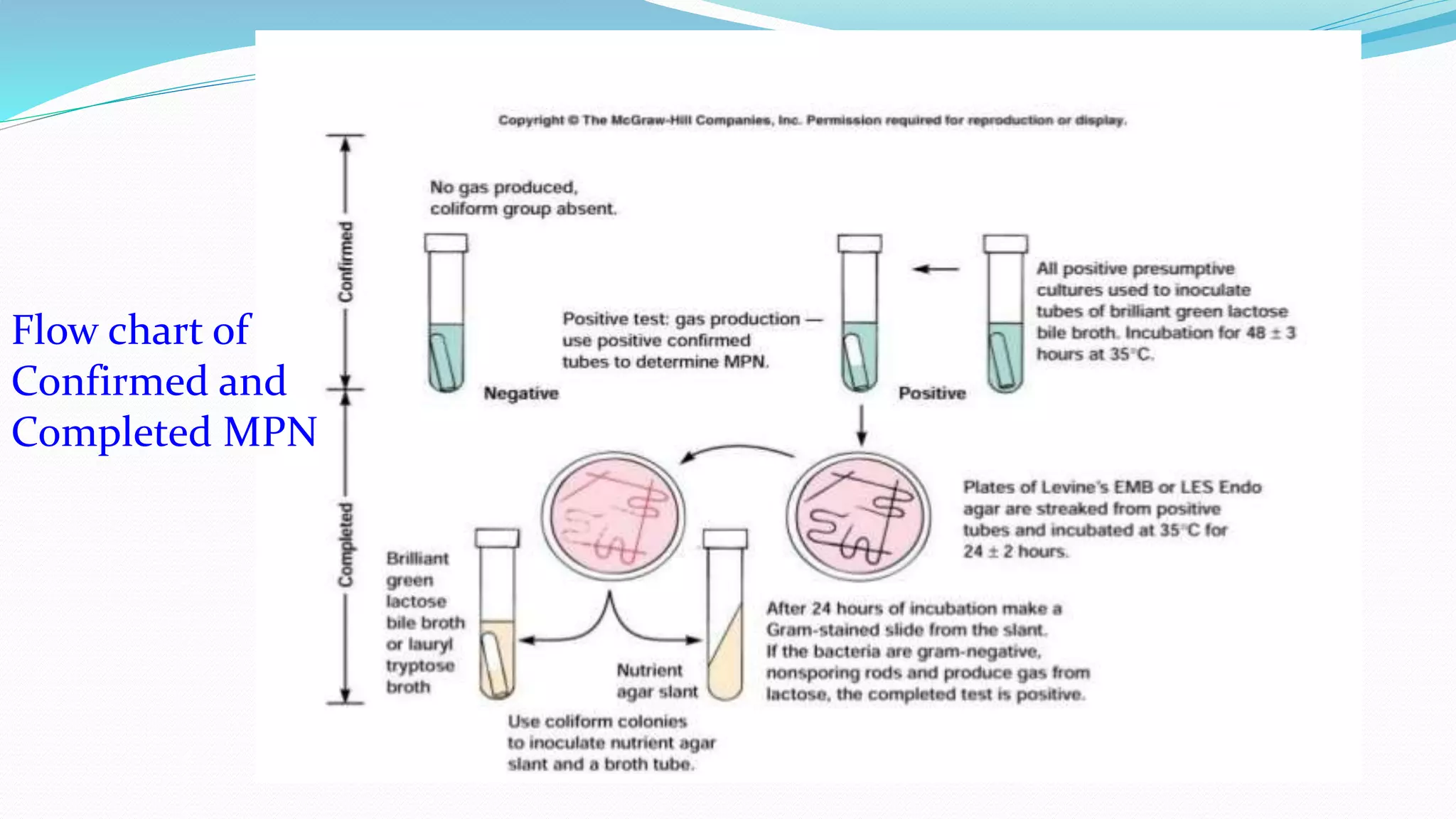
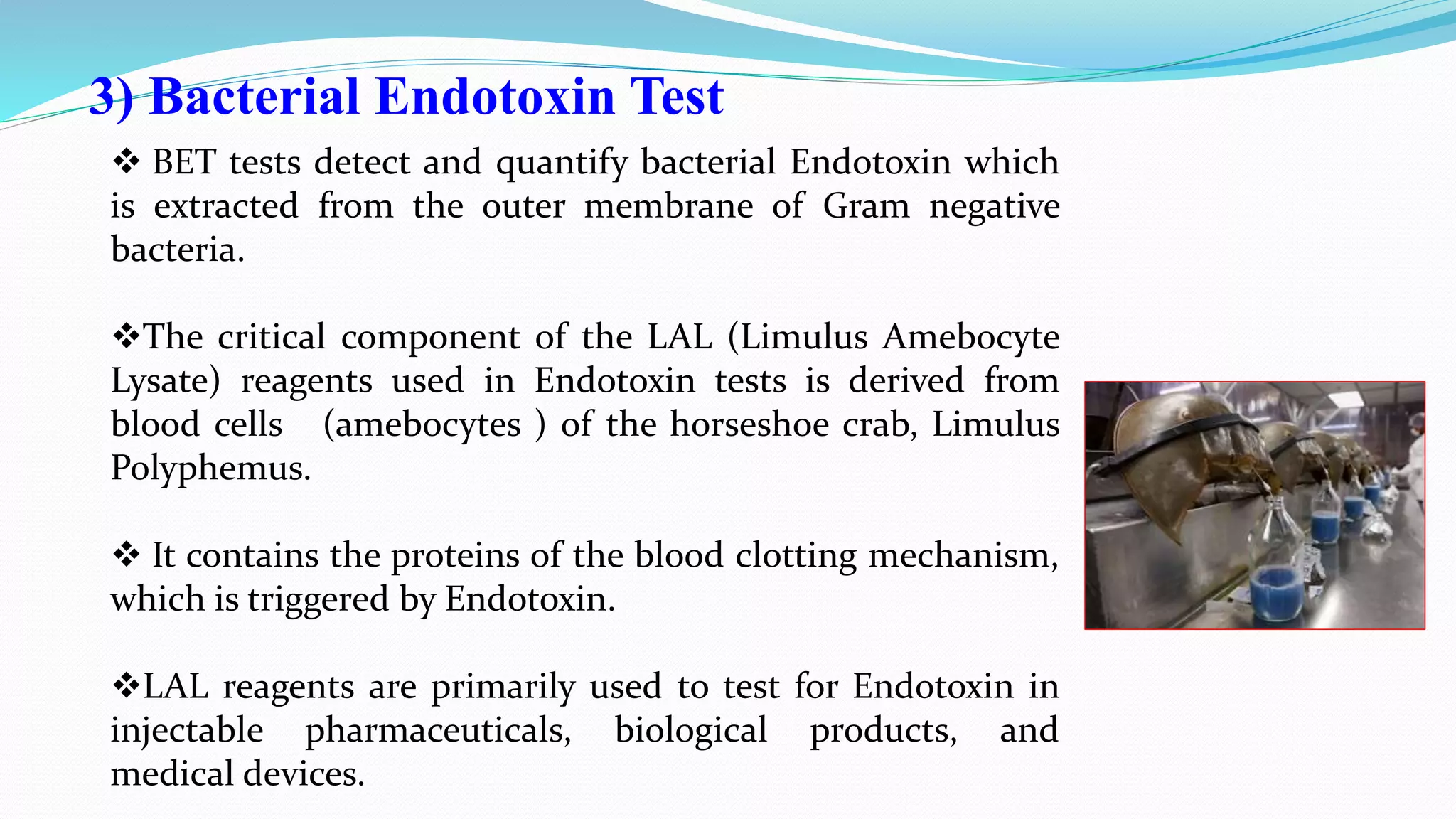
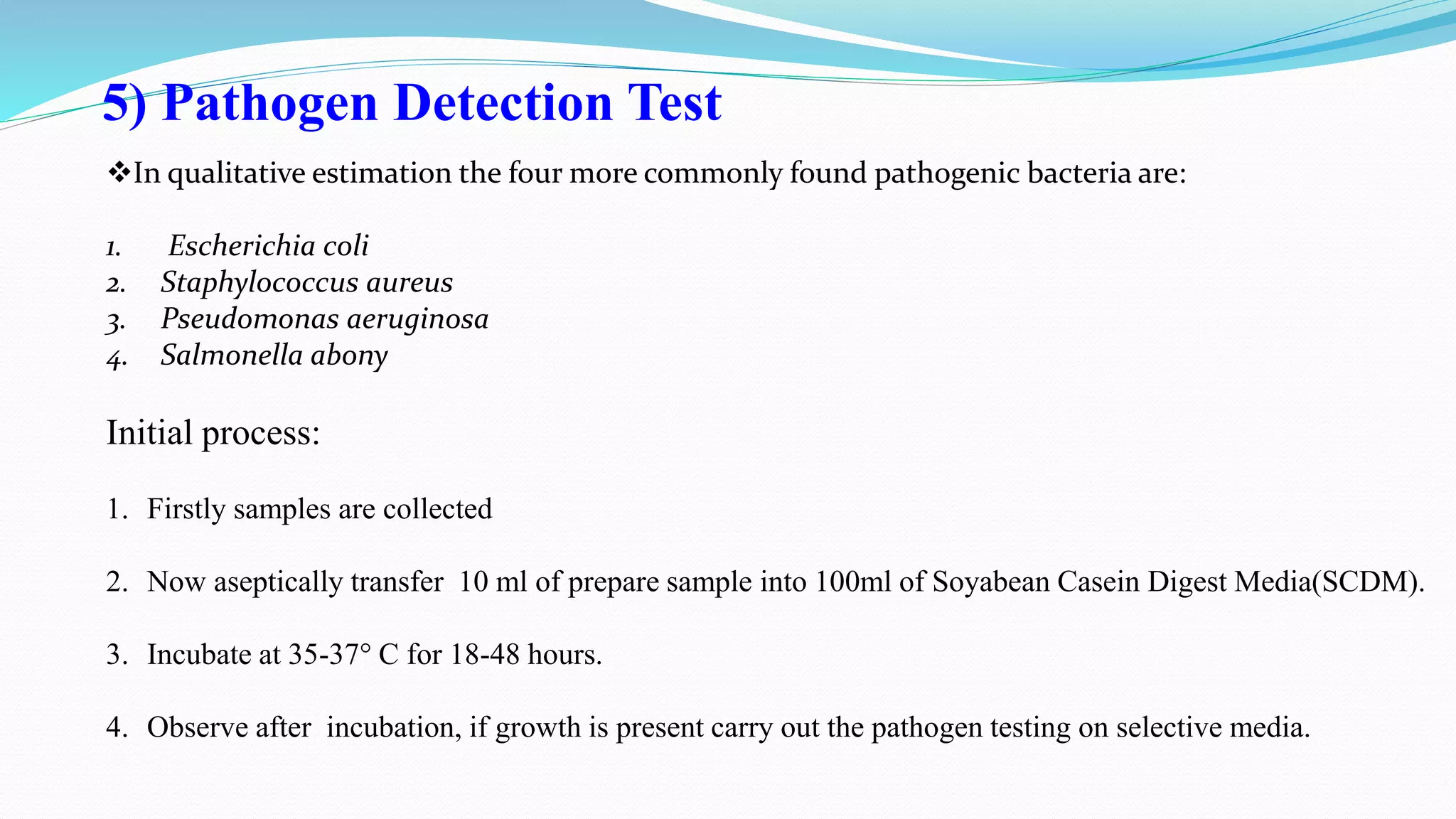
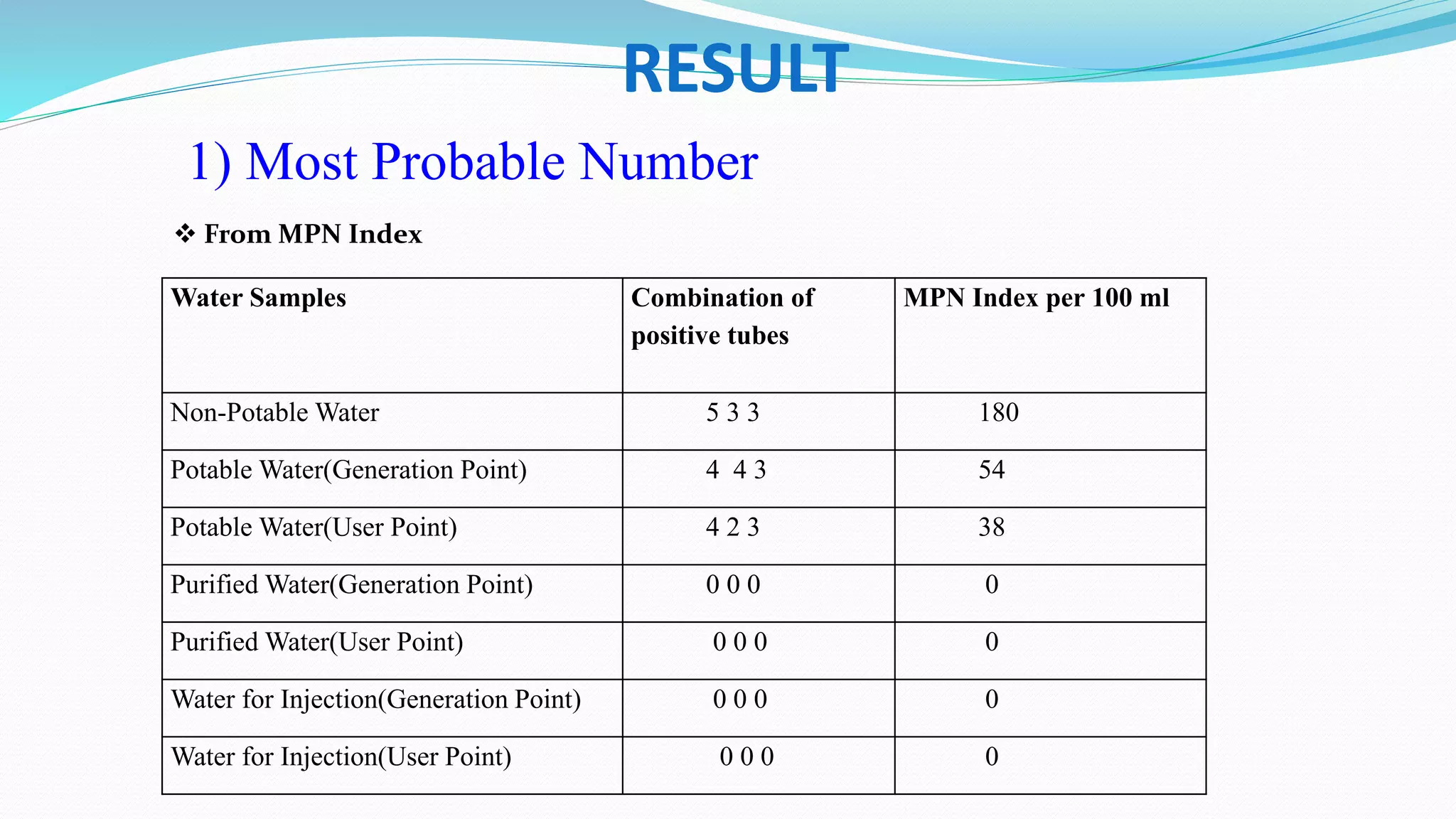
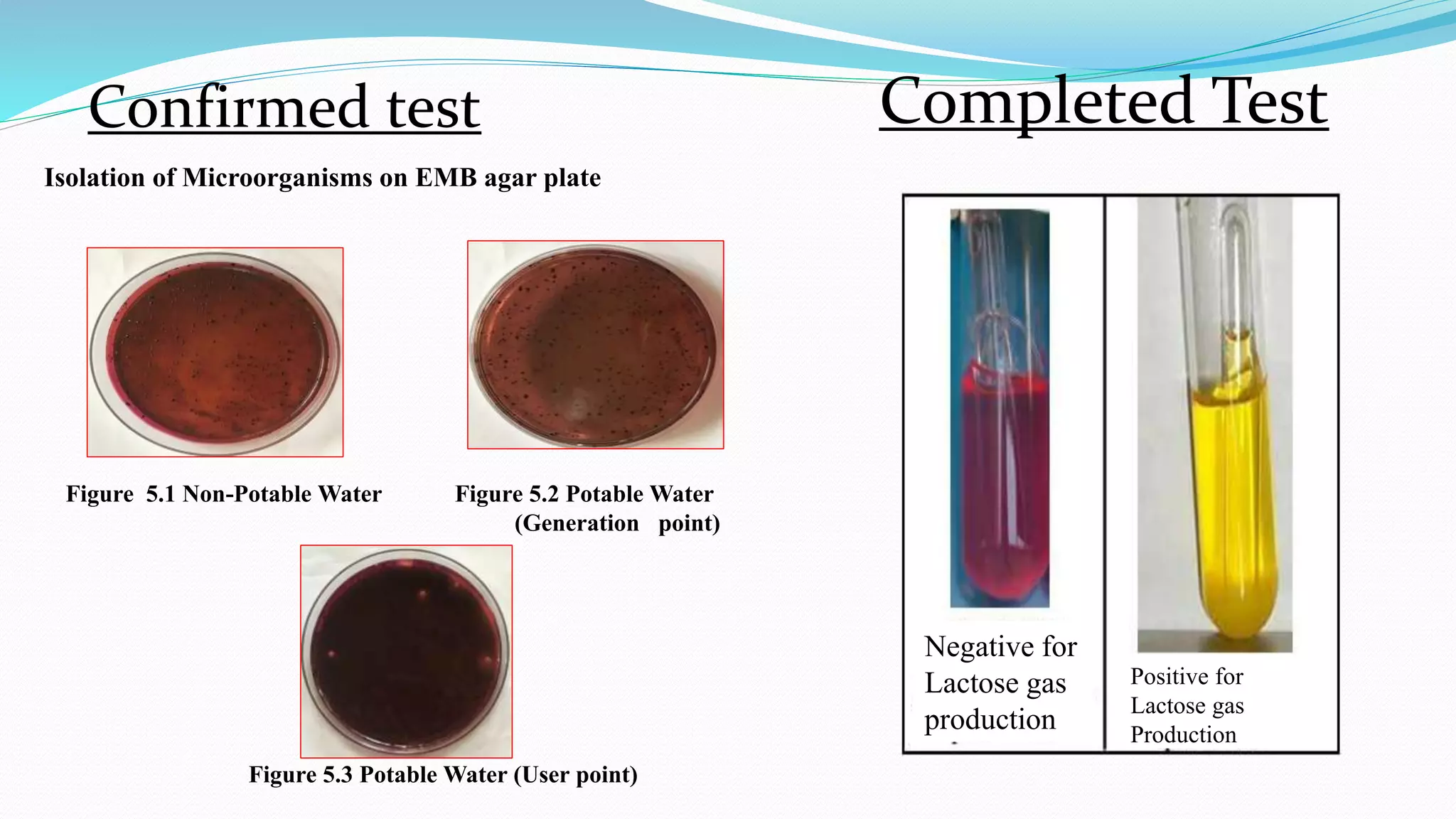
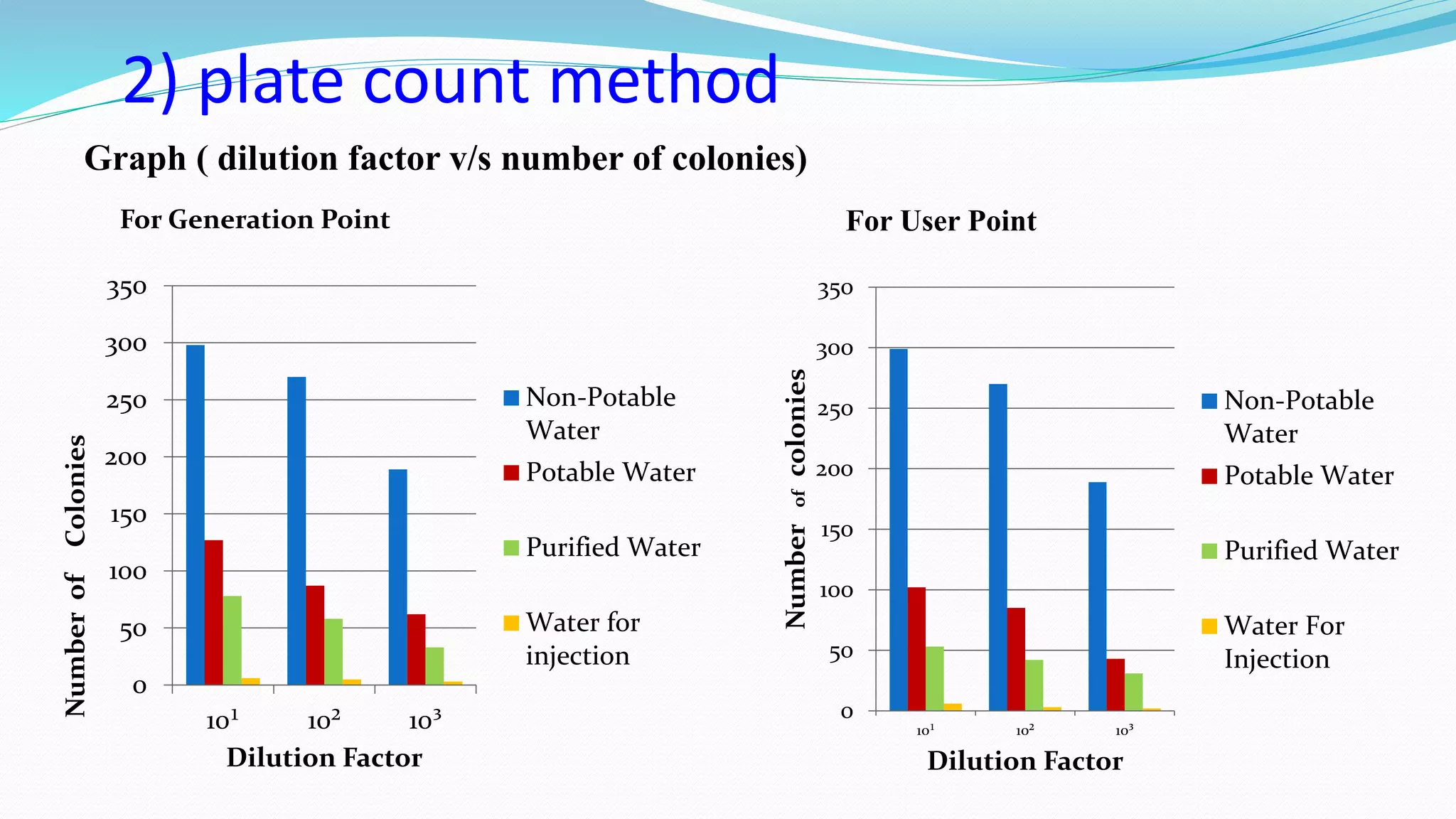
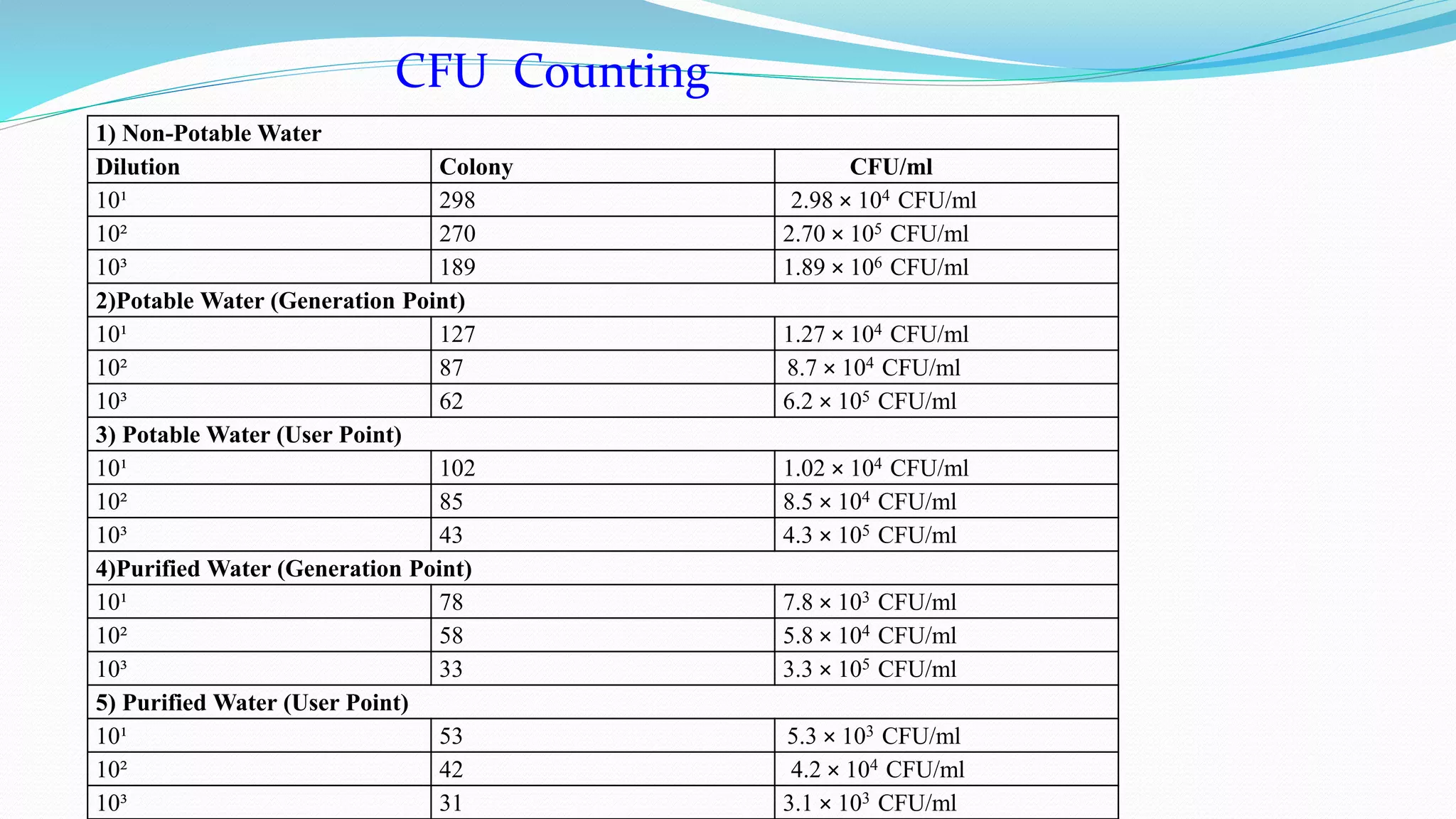
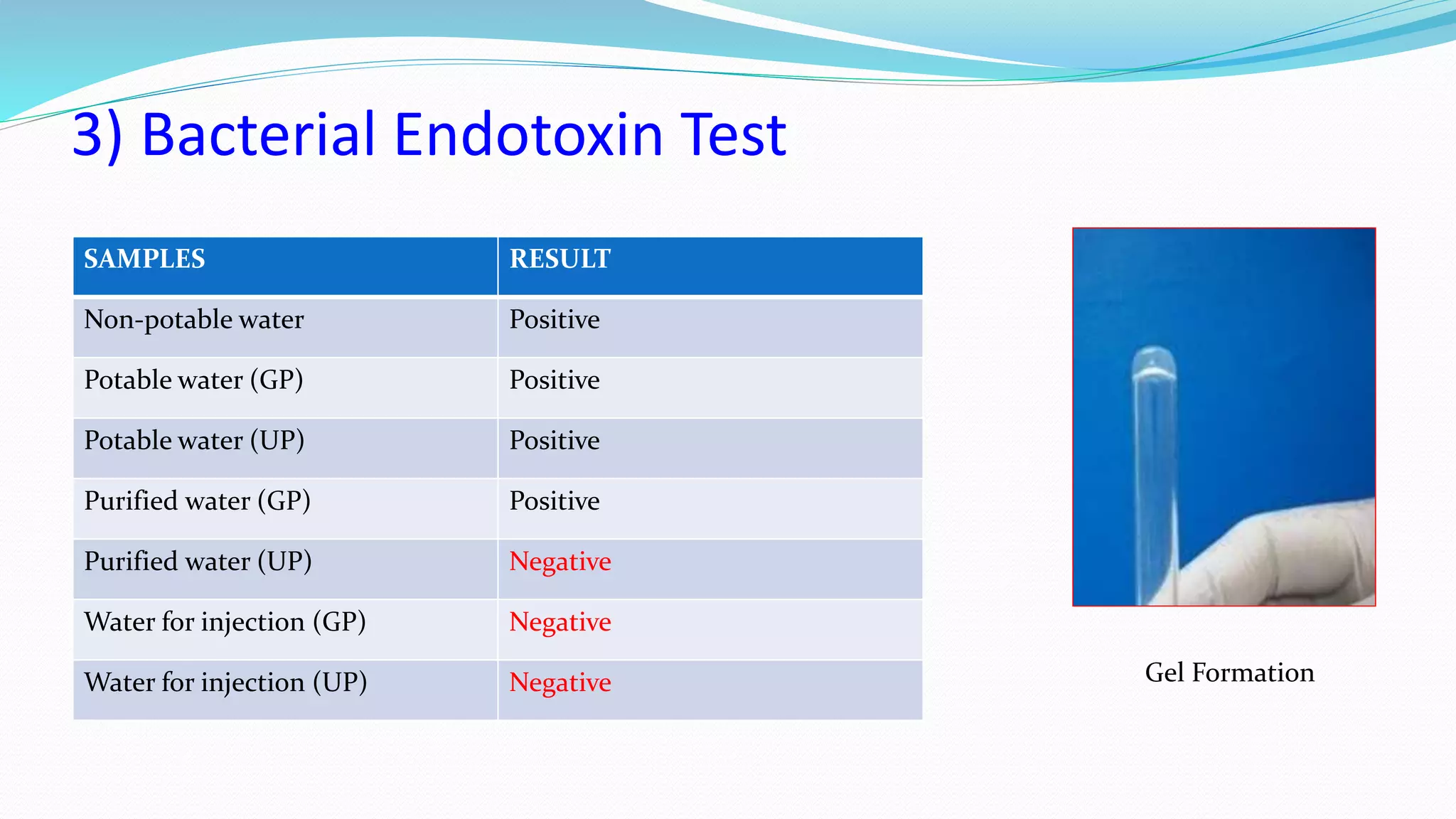
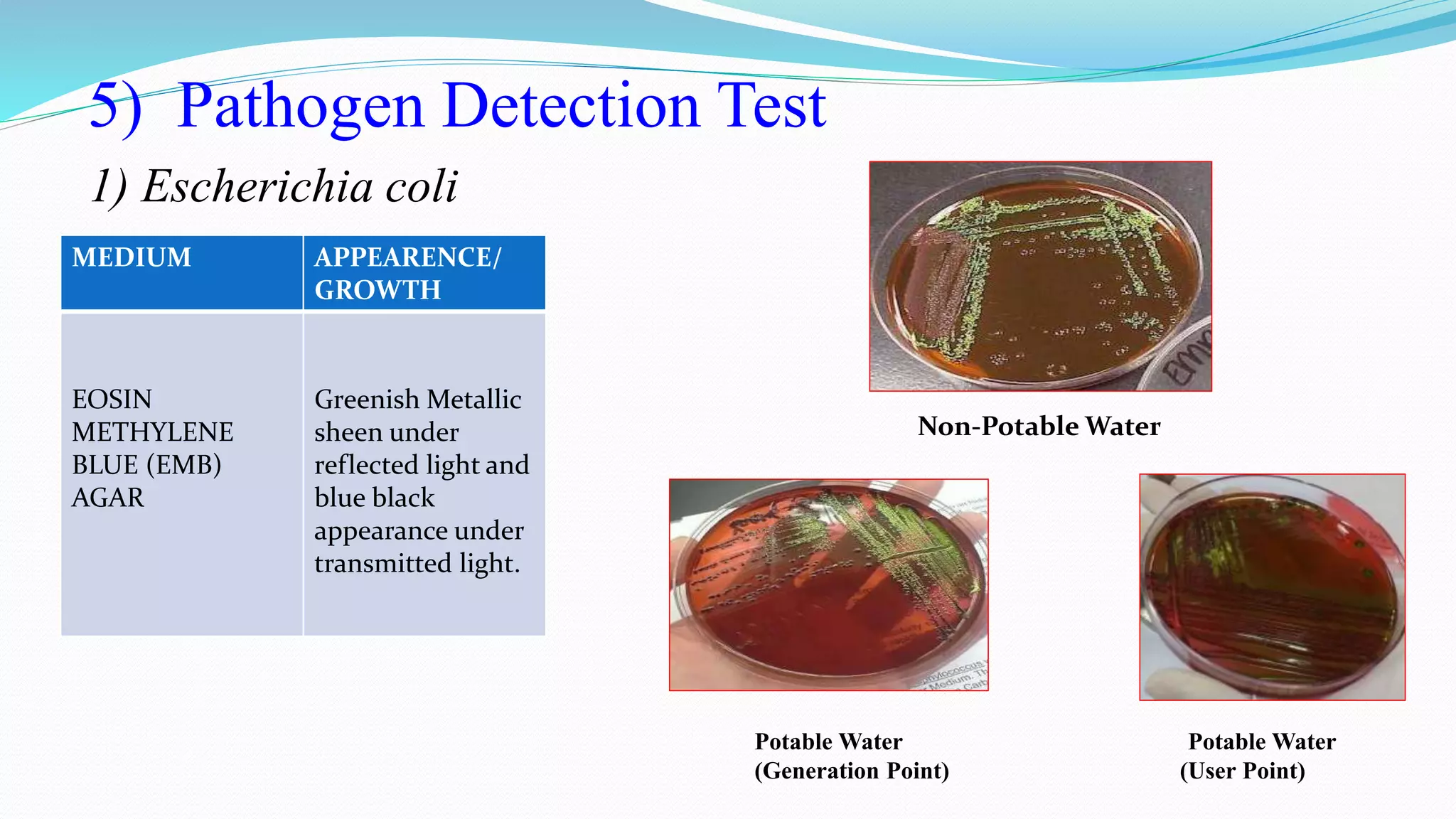
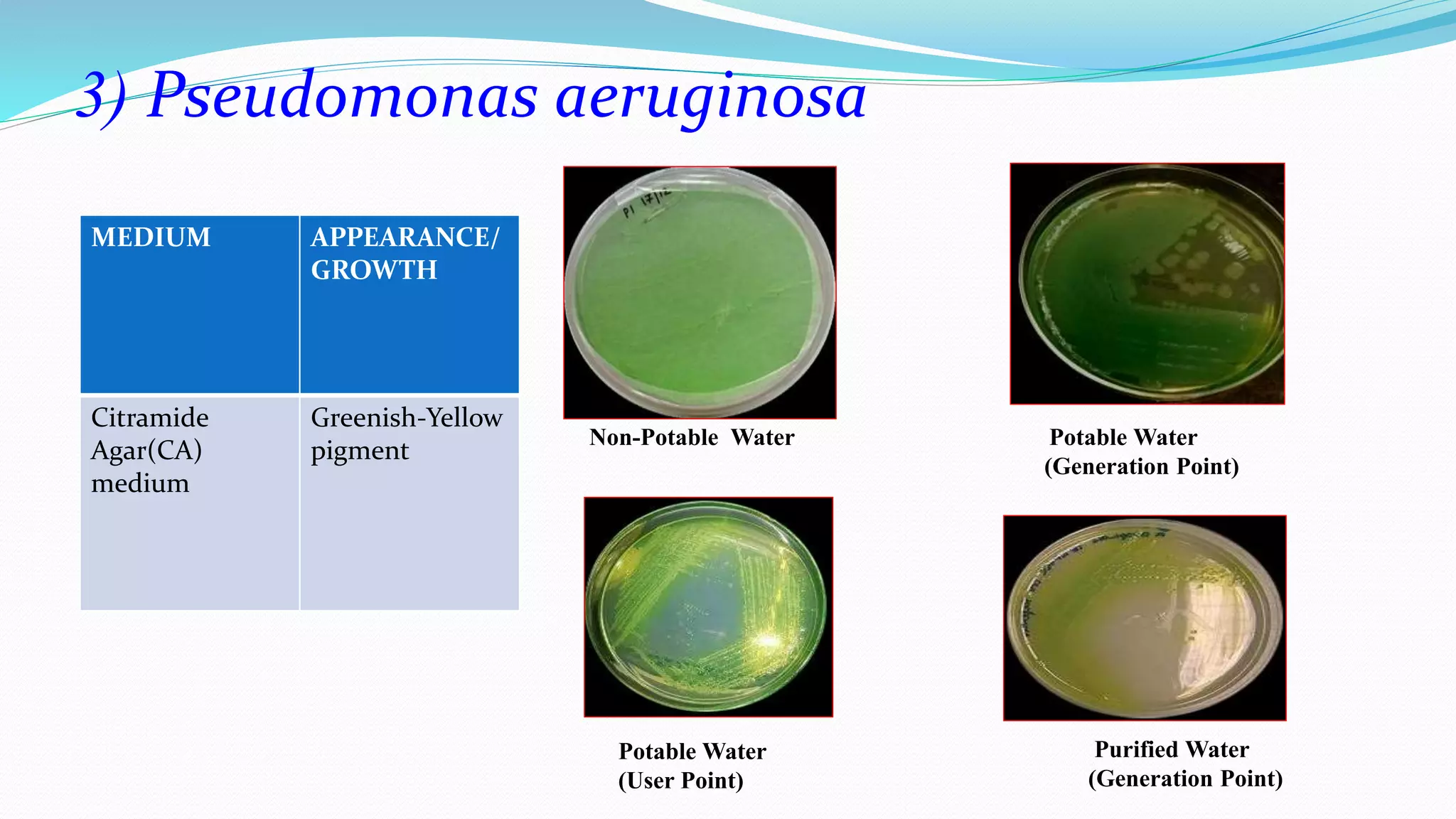
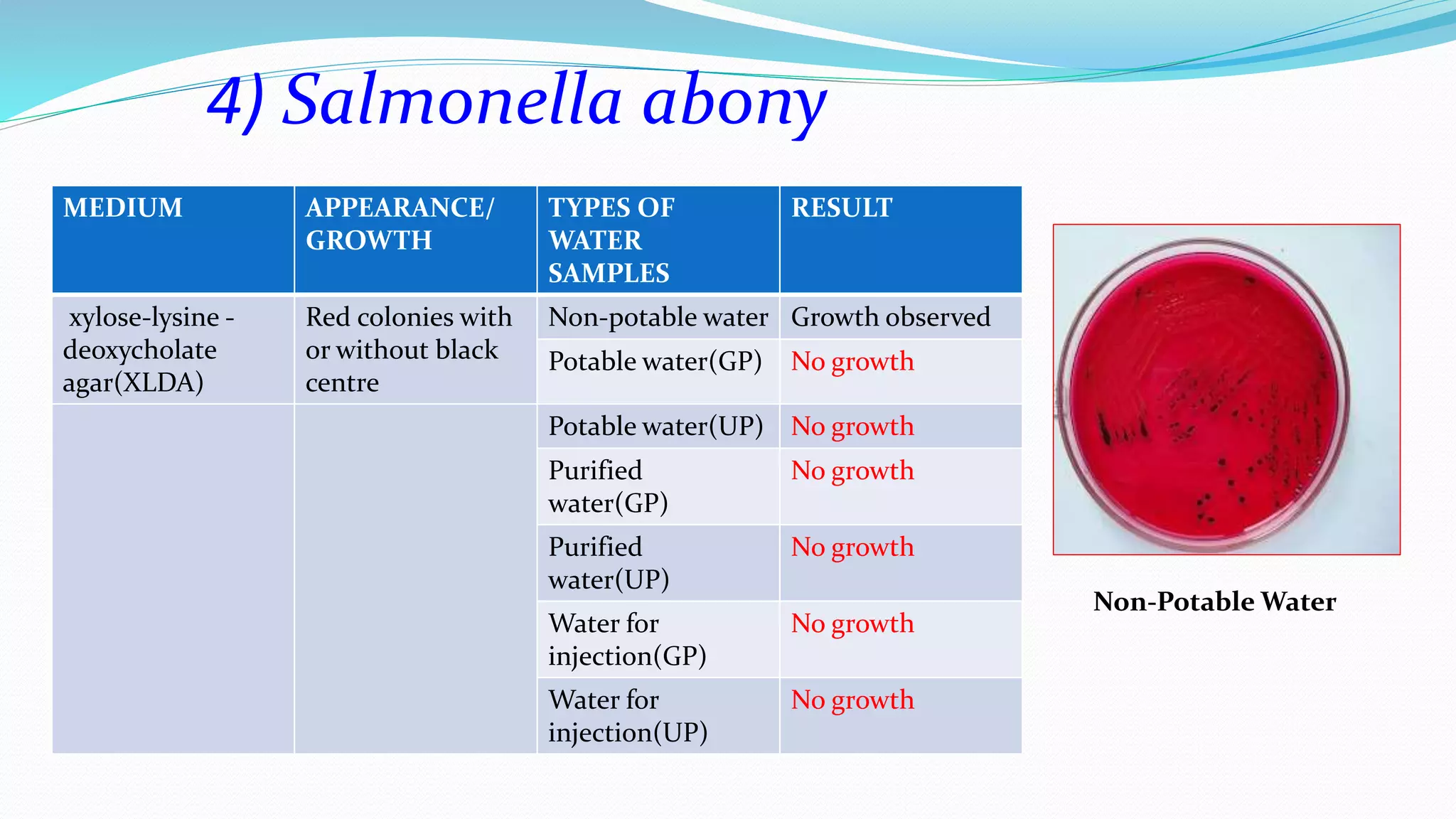
The document presents an analysis of microbial quality in various grades of water used in veterinary vaccine manufacturing, emphasizing the importance of microbiological control. It describes methods for testing water samples including microbial limit tests and bacterial endotoxin tests, highlighting potential pathogens and results from different water sources. Overall, the findings confirm that potable water, purified water, and water for injection meet microbiological safety acceptance limits essential for vaccine production.