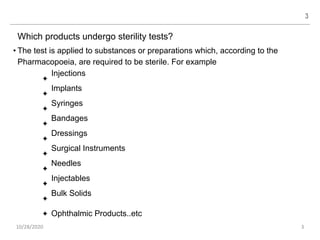
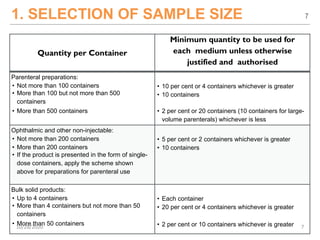
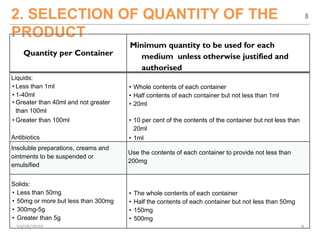
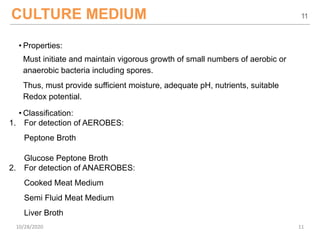
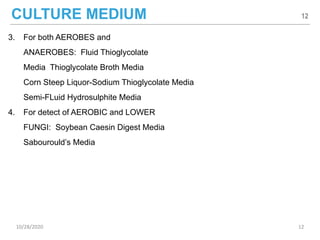
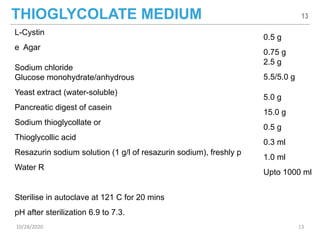
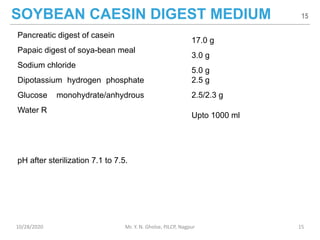
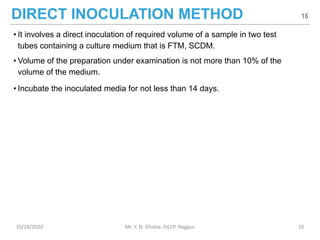
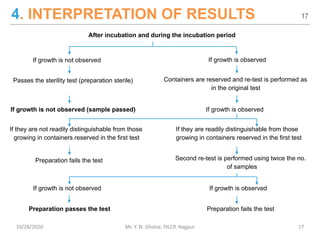
The document provides a comprehensive overview of sterility testing, which is essential for ensuring that pharmaceutical products are free from viable microorganisms. It outlines the products subject to sterility tests, precautions to prevent contamination, and specific methodologies like membrane filtration and direct inoculation methods. Additionally, it details the selection of sample sizes, quantities, and interpretation of results following incubation.