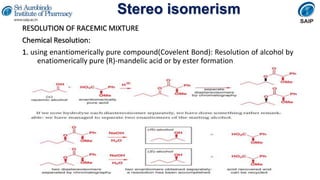
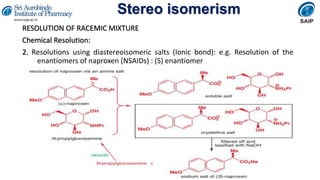
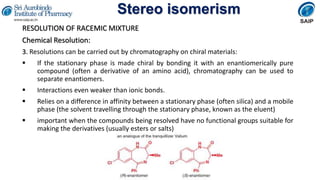
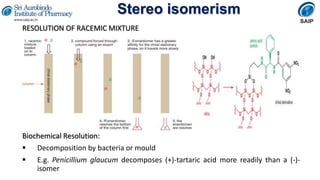
This document provides an overview of stereoisomerism and chiral chemistry. It discusses key topics such as:
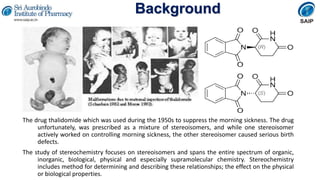
- The thalidomide disaster which showed that stereoisomers can have different biological effects.
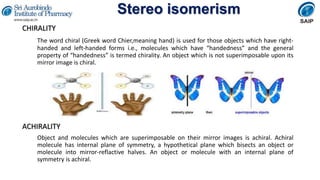
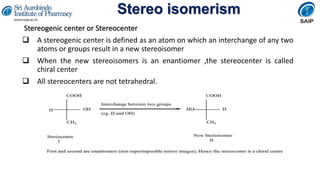
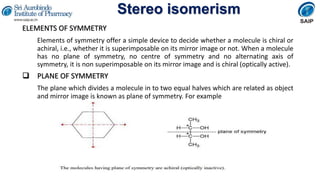
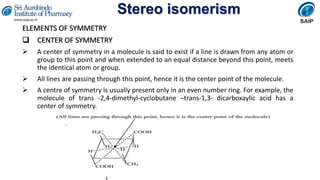
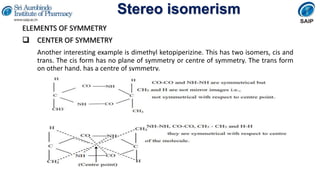
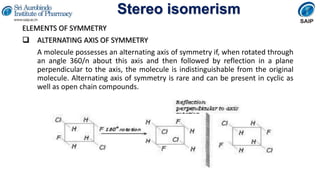
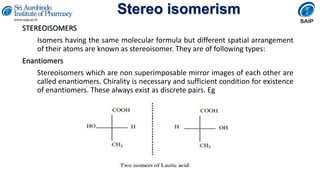
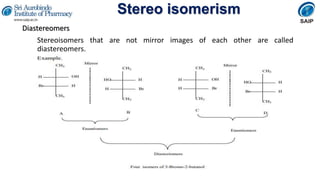
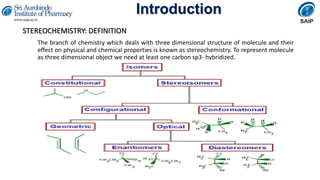
- Definitions of stereochemistry, chirality, stereoisomers including enantiomers and diastereomers.
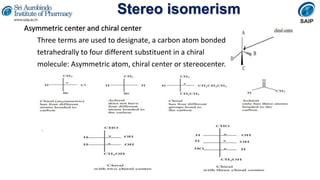
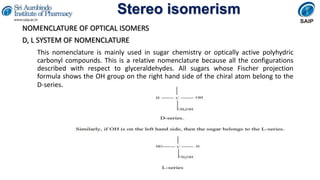
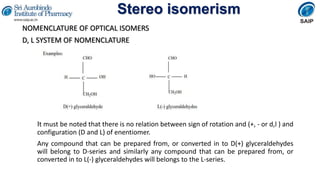
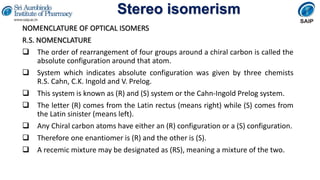
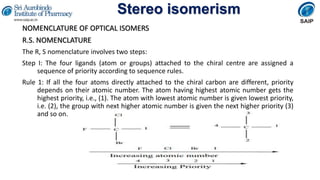
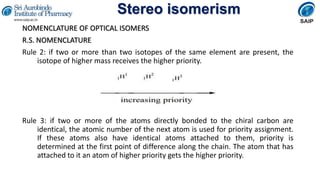
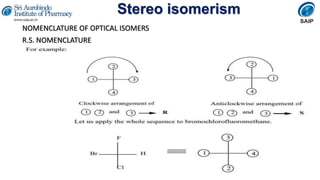
- Methods for determining and describing stereochemistry including R/S nomenclature.
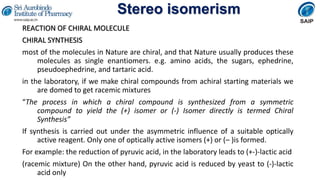
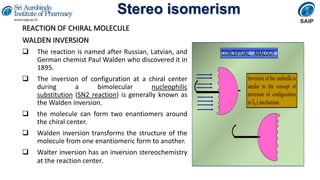
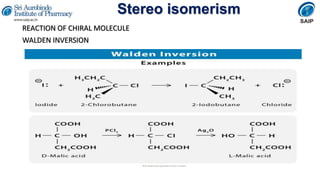
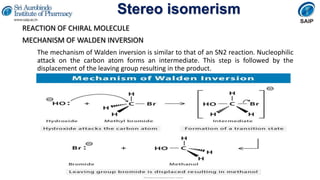
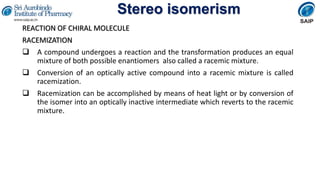
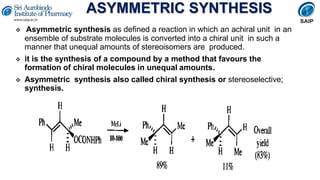
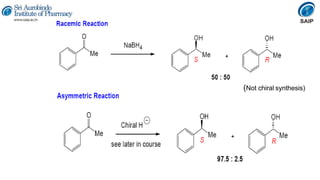
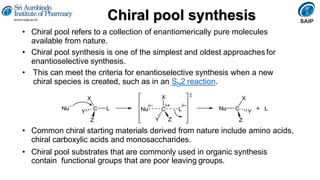
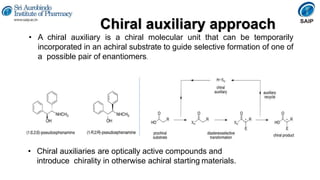
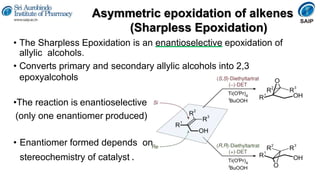
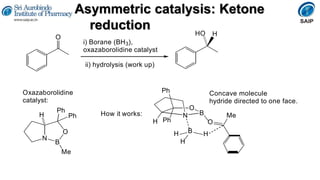
- Reactions involving chiral molecules such as asymmetric synthesis, resolution of racemic mixtures, and Walden inversion.

![Stereo isomerism
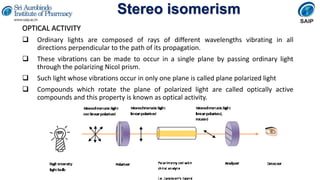
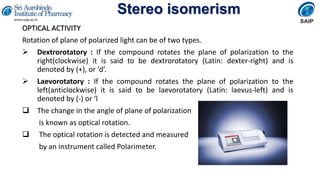
OPTICAL ACTIVITY
The measurement of optical activity is reported in terms of specific rotation
[α], which is given as,
[α] λ t = α/lc
[α]= specific rotation
t = temperature of measurement
λ=wavelength of the light used
α= observed angle of rotation
l= length of sample tube in decimeter
c=concentration of the sample in g/mL of solution](https://image.slidesharecdn.com/stereoisimerism-210324070846/85/Stereo-isomerism-6-320.jpg)