The document provides information about an upcoming presentation on Good Clinical Practice (GCP). It includes:
1) Details about the presenter including his background and experience in clinical research.
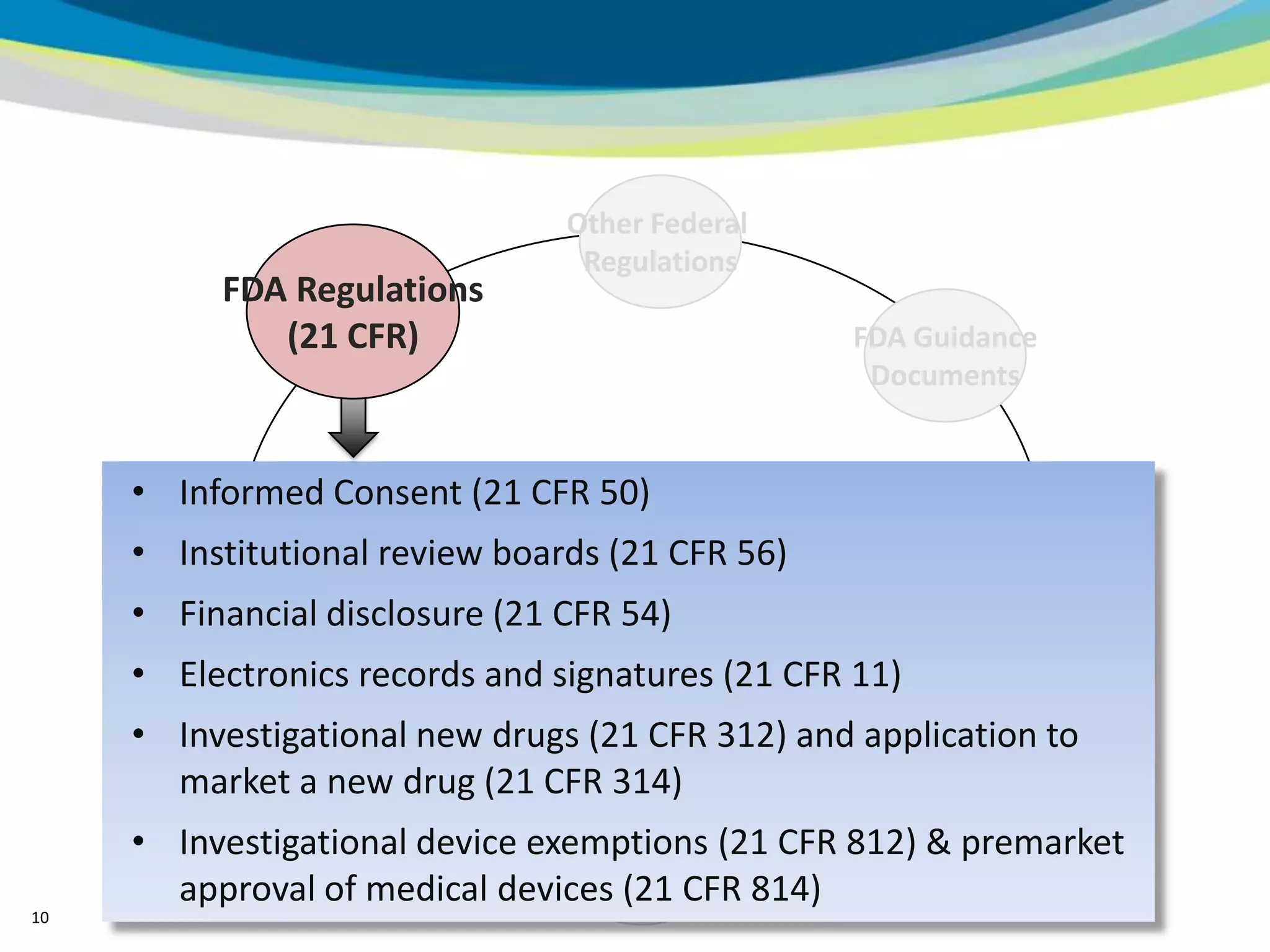
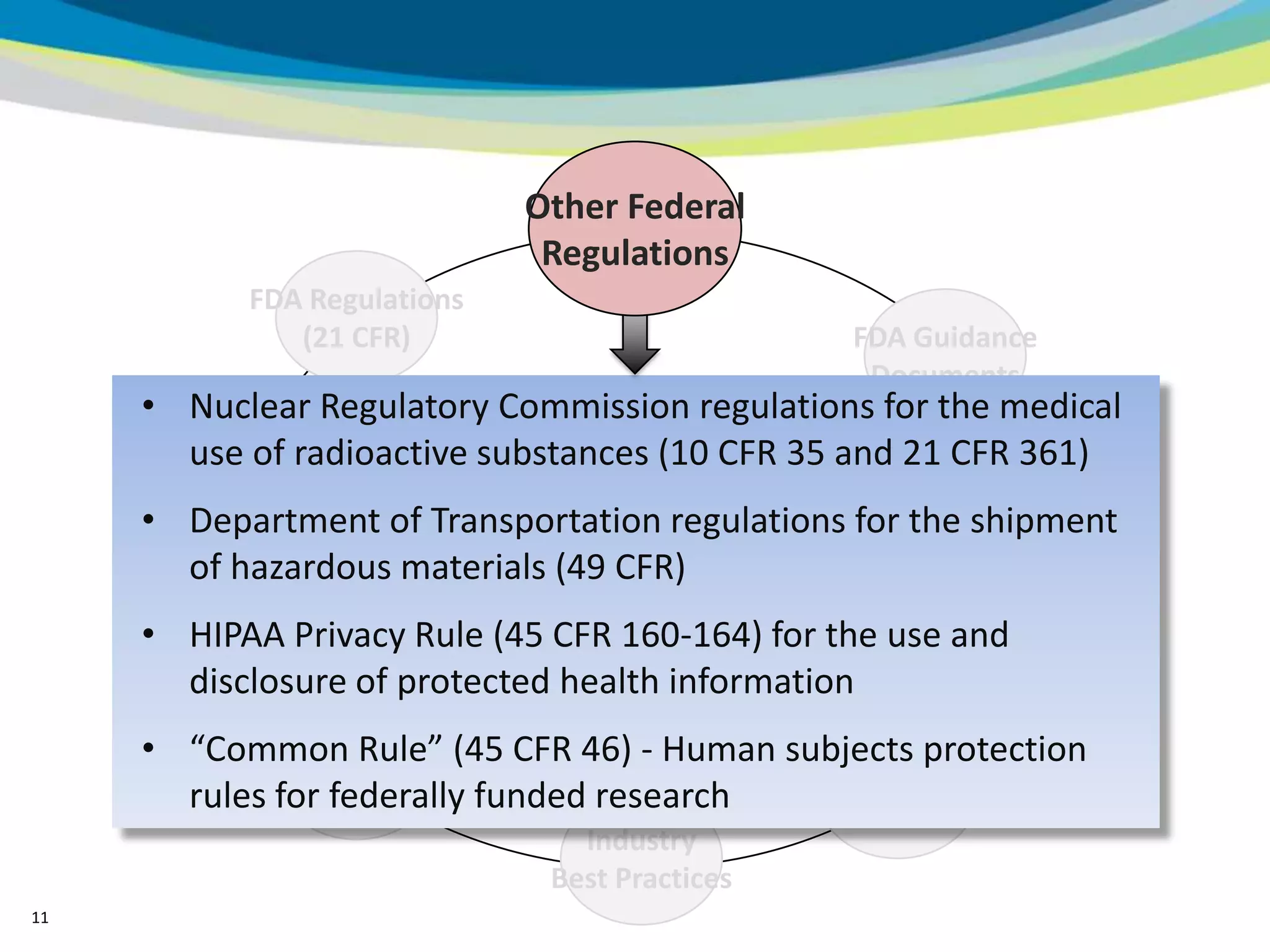
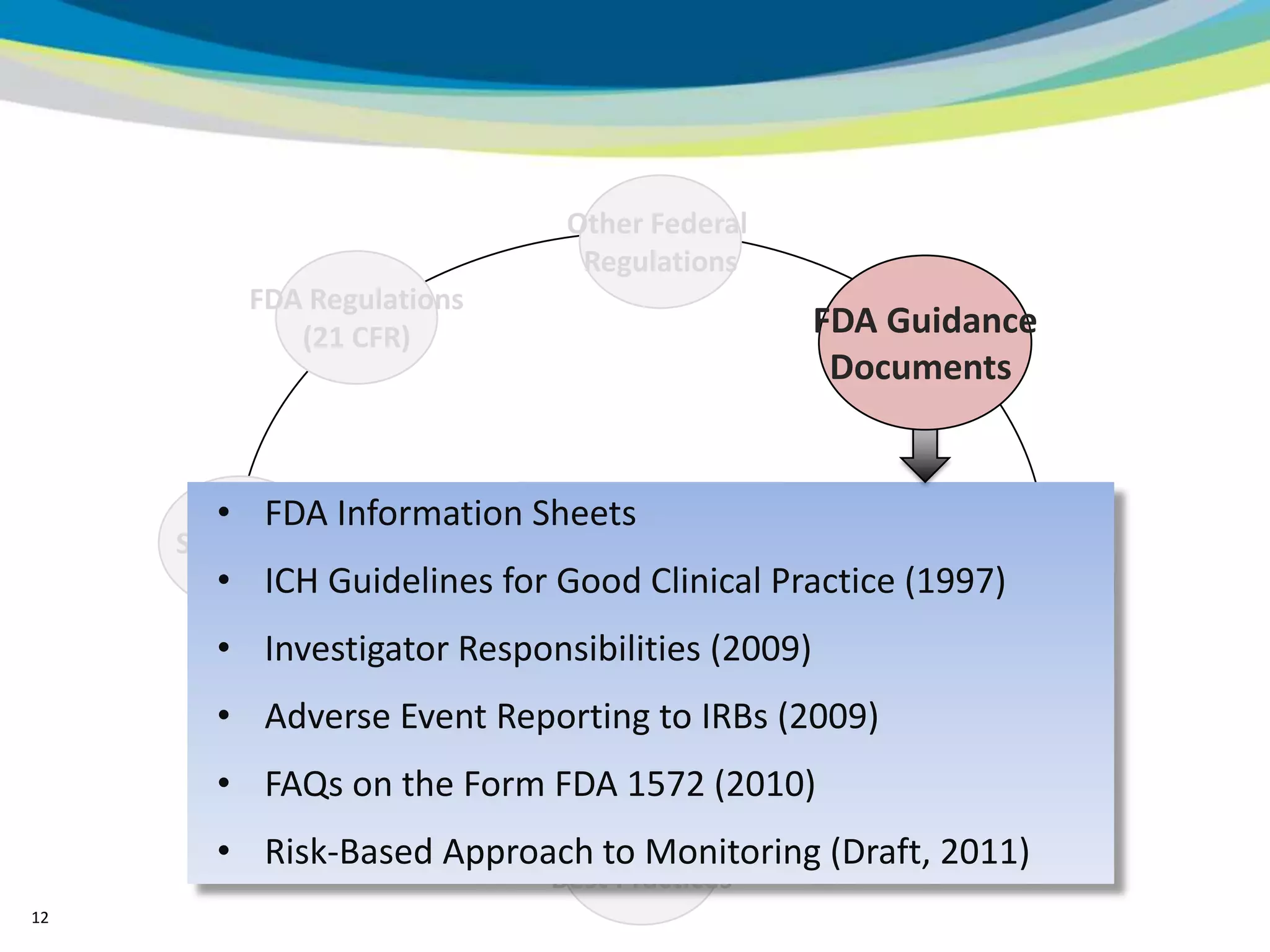
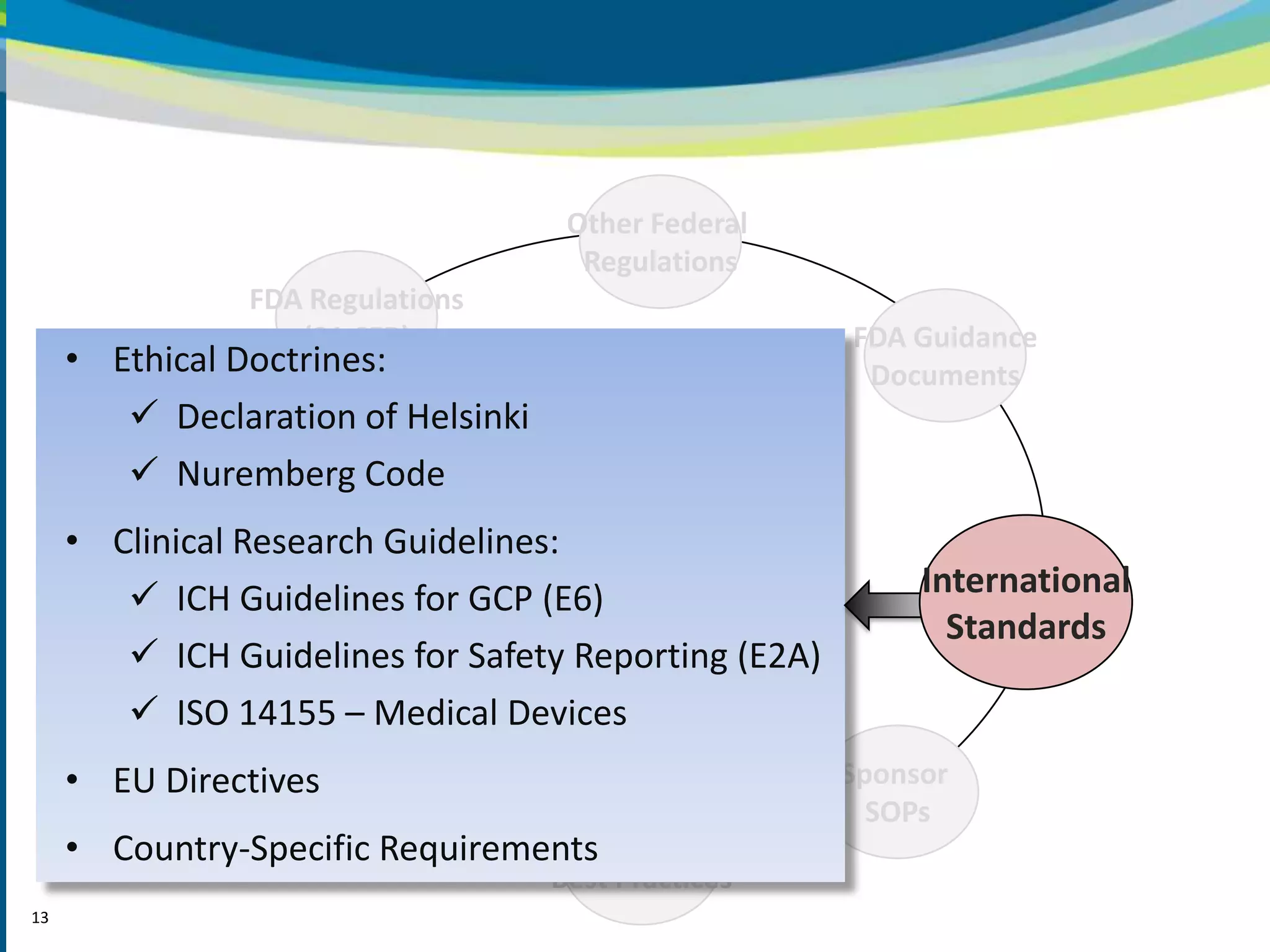
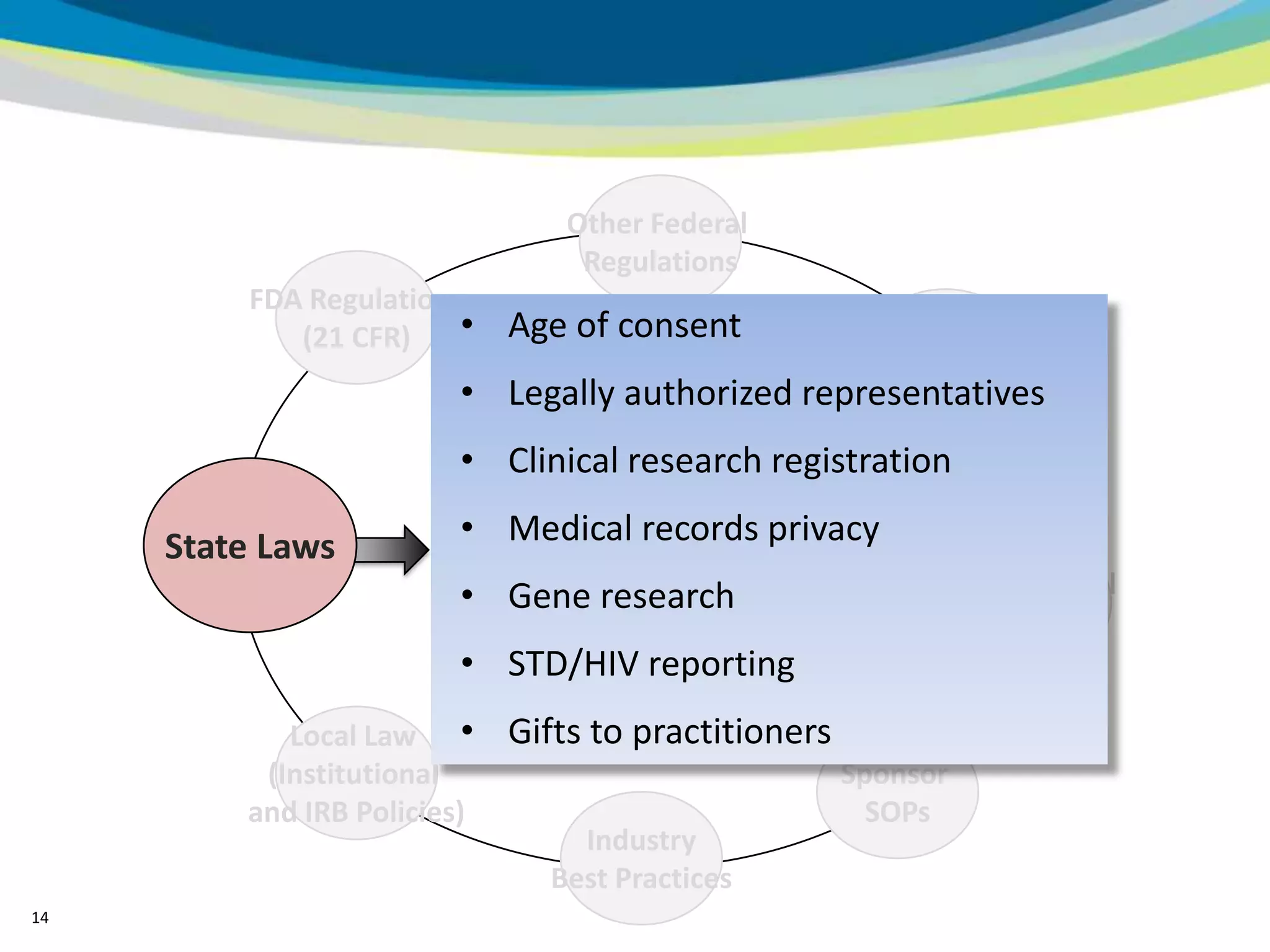
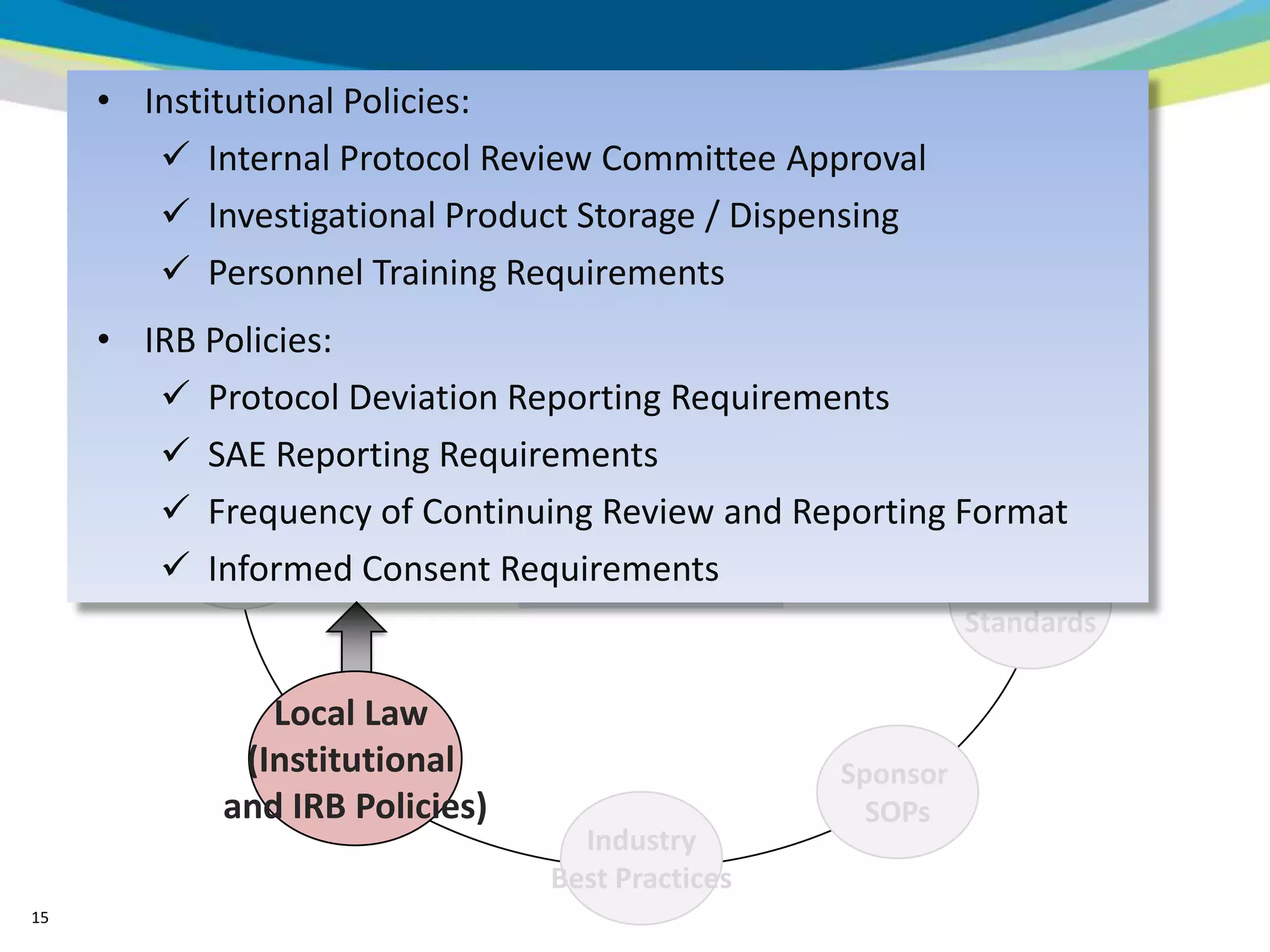
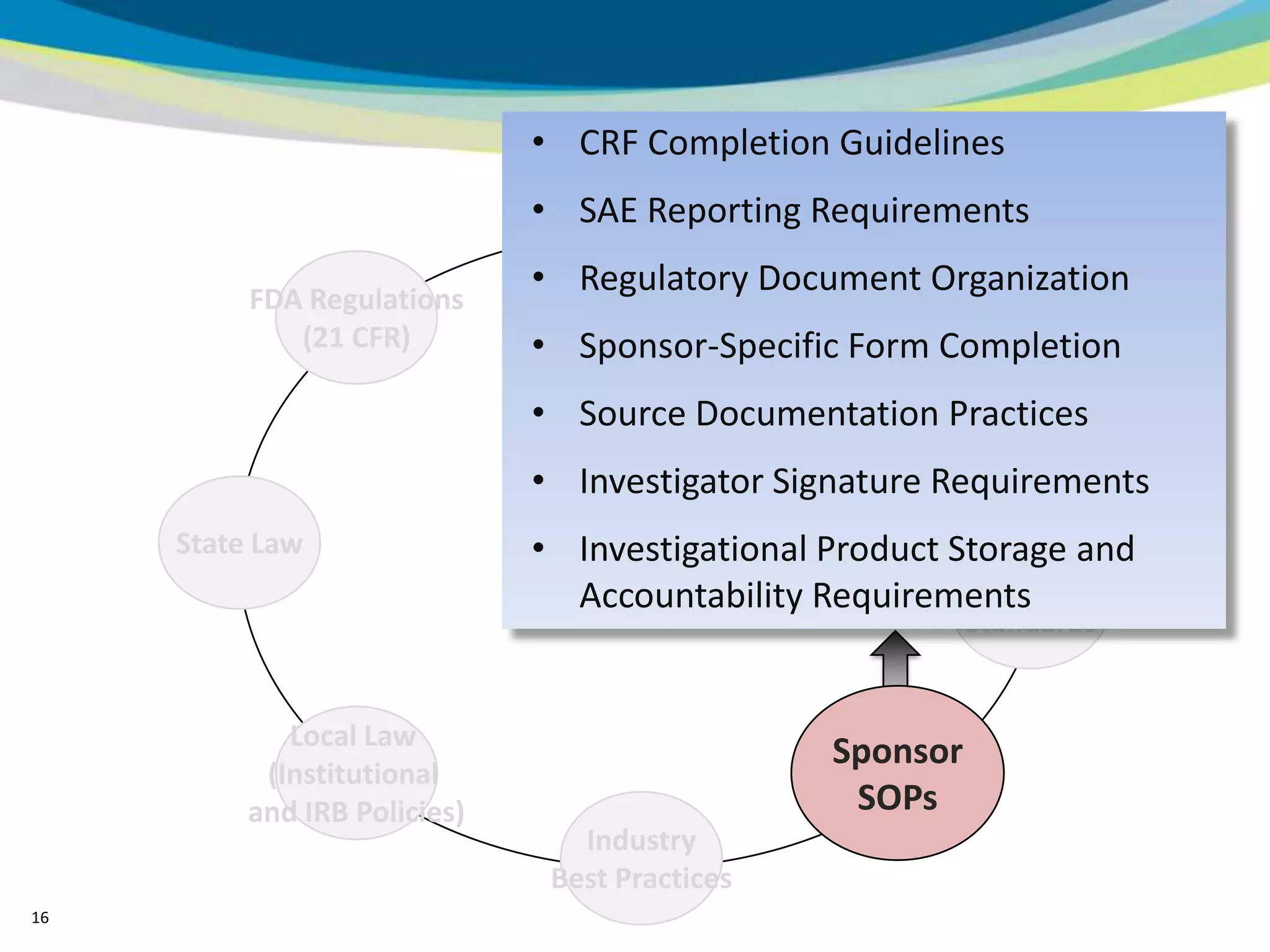
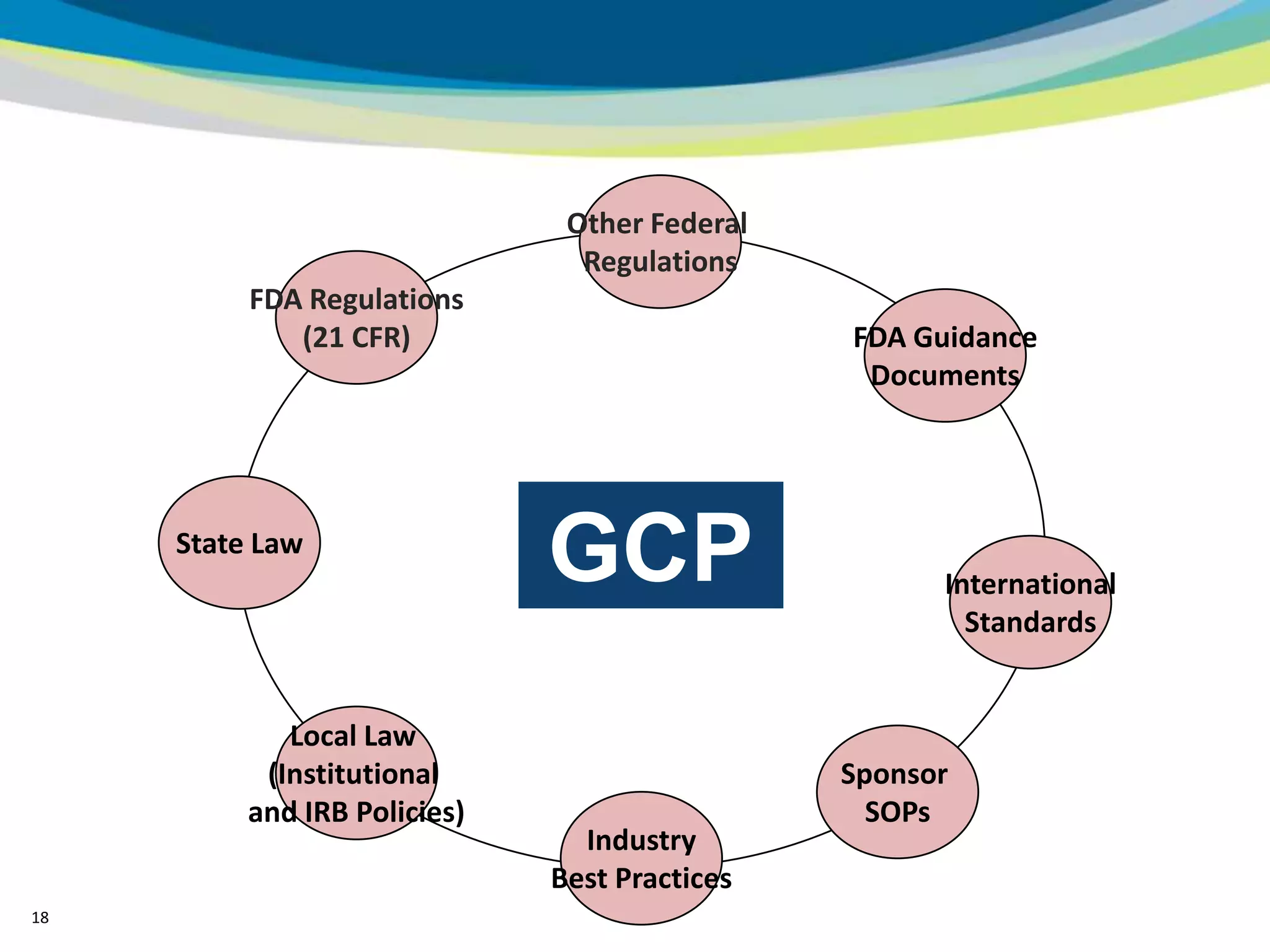
2) The learning objectives of the presentation which are to define GCP, differentiate GCP requirements from recommendations, and identify circumstances where industry best practices go beyond FDA requirements.
3) An introduction stating the presentation was developed to correct common myths and errors heard about GCP throughout the presenter's career.