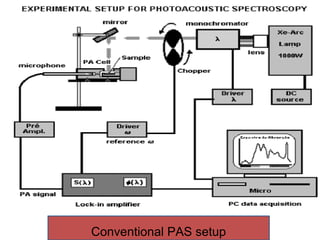
This document provides an overview of photoacoustic spectroscopy (PAS). It discusses the history of PAS, which was discovered by Alexander Graham Bell in 1880. PAS allows for absorption spectroscopy of opaque and scattering samples by detecting the acoustic signal generated from the sample's absorption of modulated light. The document describes the basic photoacoustic effect, conventional PAS setups, applications like surface studies and blood analysis, and examples of PAS spectra. It establishes PAS as a non-destructive technique for obtaining optical absorption data from solids, semisolids, and turbid liquids that conventional spectroscopy cannot analyze due to light scattering.