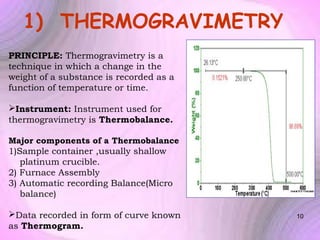
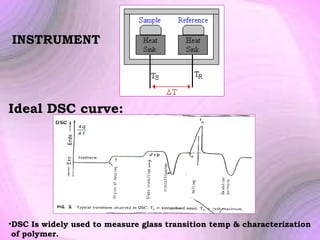
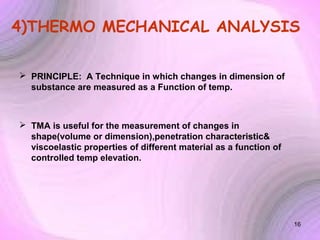
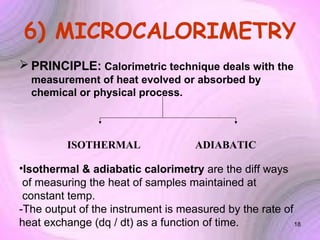
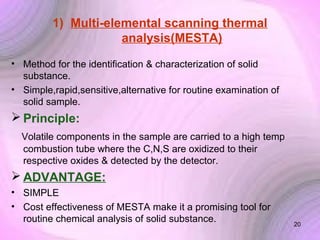
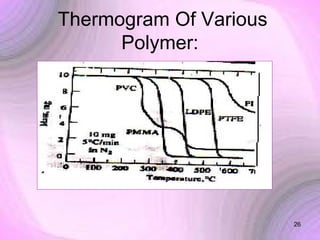
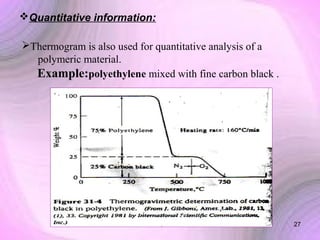
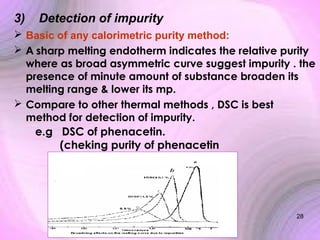
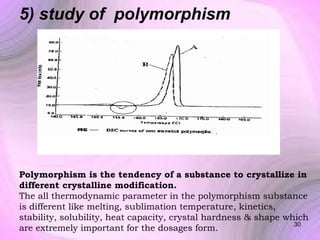
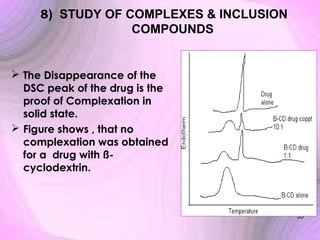
This document summarizes a seminar presentation on preformulation studies using thermal analysis, X-ray diffraction, and FT-IR spectroscopy. The presentation discusses the role of these techniques in preformulation, including methods like thermogravimetry, differential thermal analysis, and differential scanning calorimetry. Applications described are polymorphism analysis, detection of impurities, drug-excipient compatibility testing, and prediction of drug stability from thermal degradation profiles. The document provides an overview of the principles and applications of various thermal analysis techniques in pharmaceutical preformulation studies.