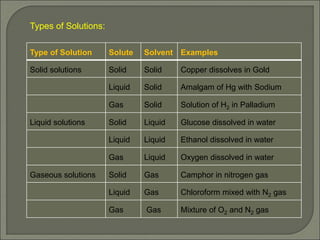
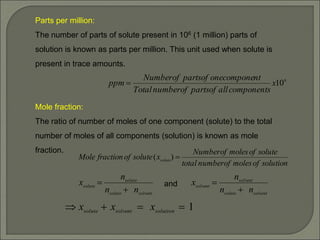
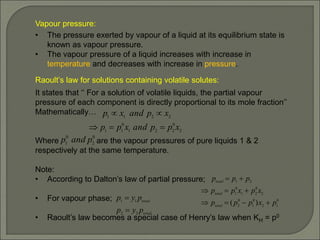
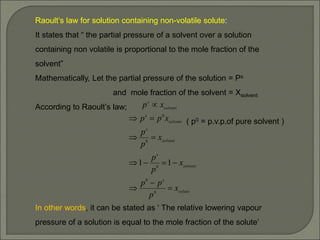
The document is a comprehensive presentation on solutions in chemistry, targeting class XII syllabus, covering definitions, types, solubility, and concentration methods. Key topics include ideal and non-ideal solutions, colligative properties, and methods for determining molar mass through various properties such as boiling point elevation and osmotic pressure. It also discusses important laws like Raoult's Law and Henry's Law, along with concepts of abnormal molar mass and the van't Hoff factor.