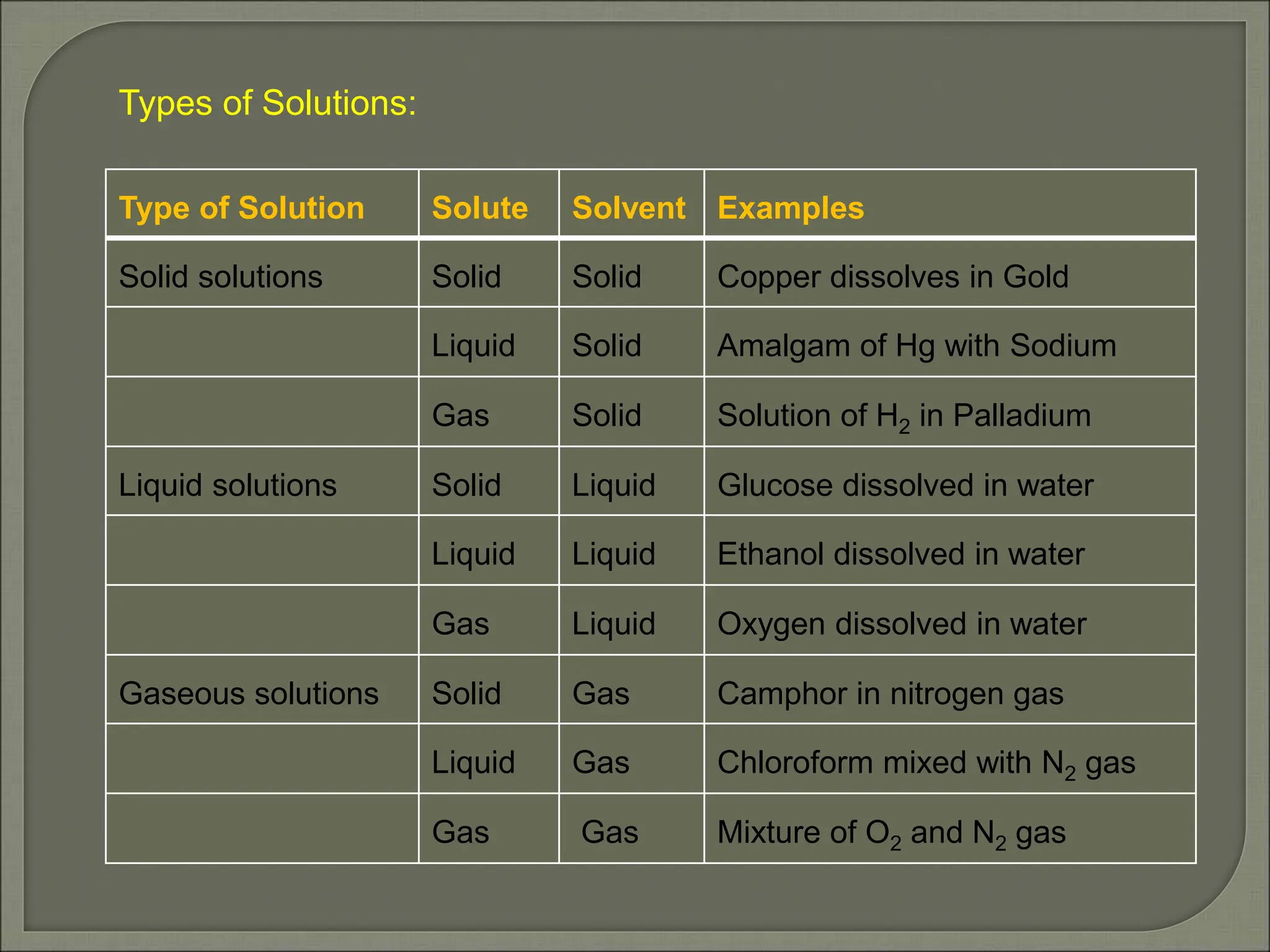
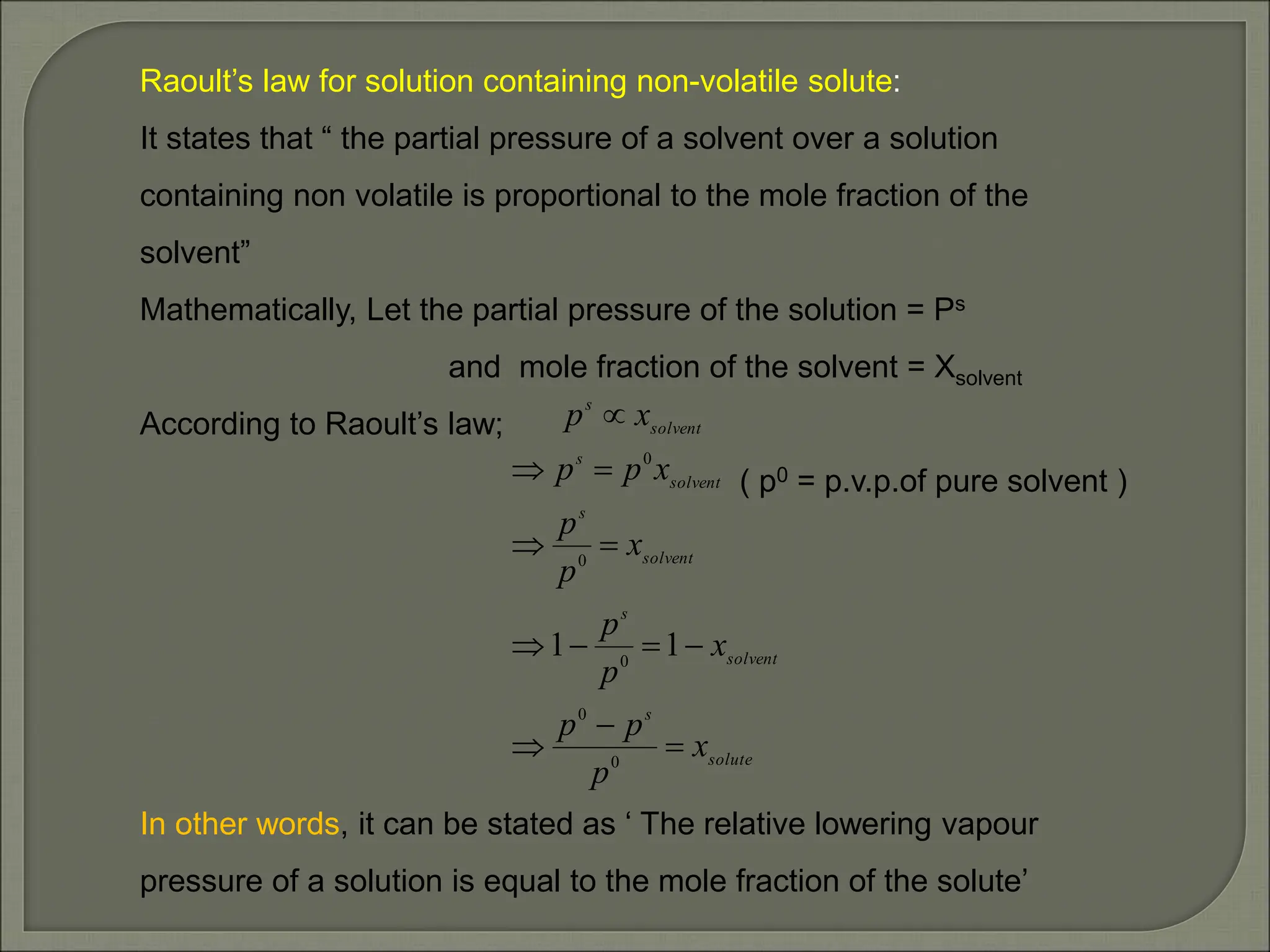
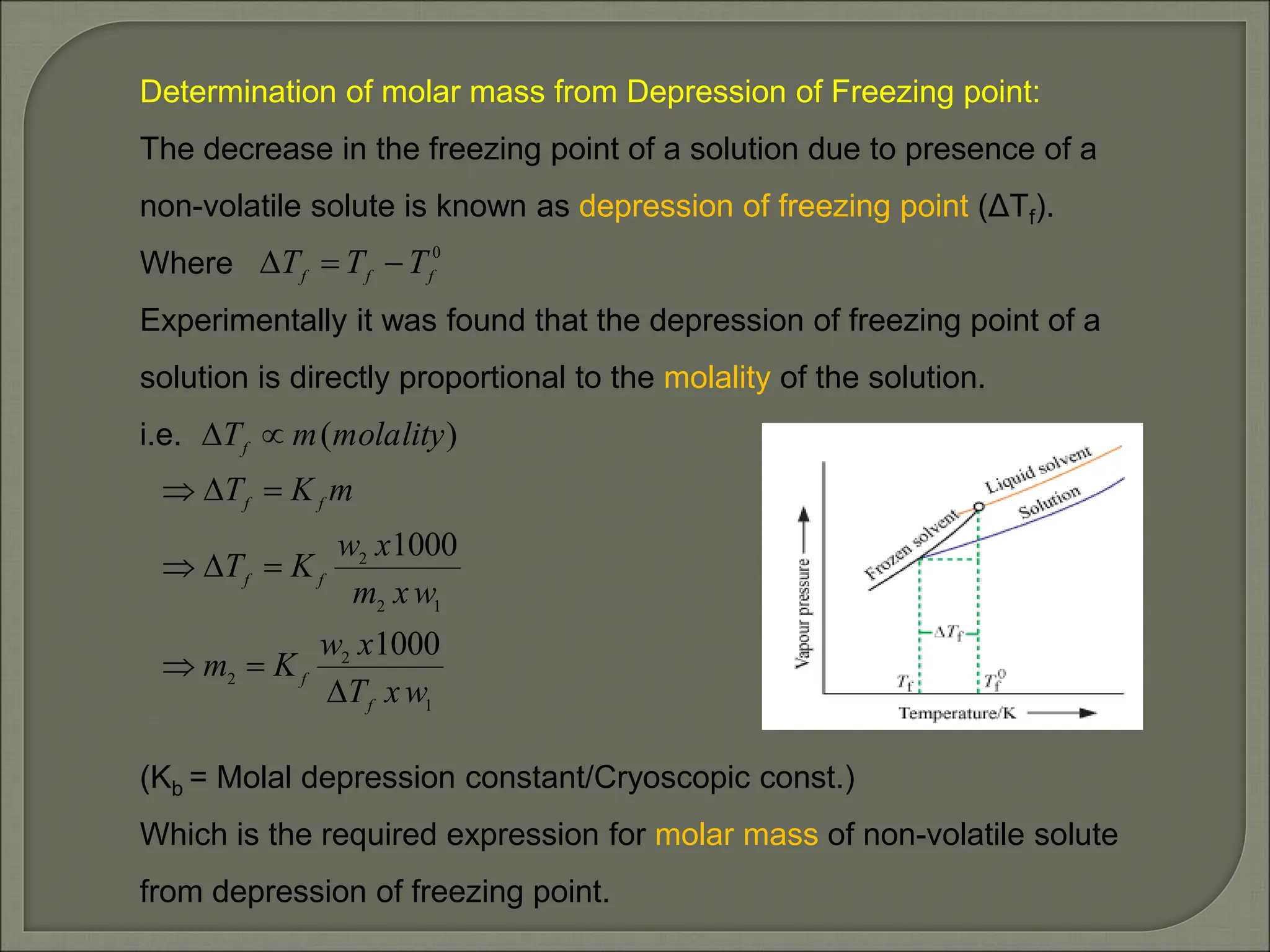
This document provides an overview of solutions and related concepts in chemistry. It begins by defining a solution as a homogeneous mixture of two or more components. It then discusses different types of solutions and various ways of expressing the concentration of solutions, including mass percentage, molarity, and molality. Key concepts covered include solubility, Raoult's law, ideal and non-ideal solutions, and azeotropes. Vapour pressure and Henry's law are also summarized in relation to gas solubility in liquids. The document aims to cover important learning objectives about solutions for a class 12 chemistry course in India.