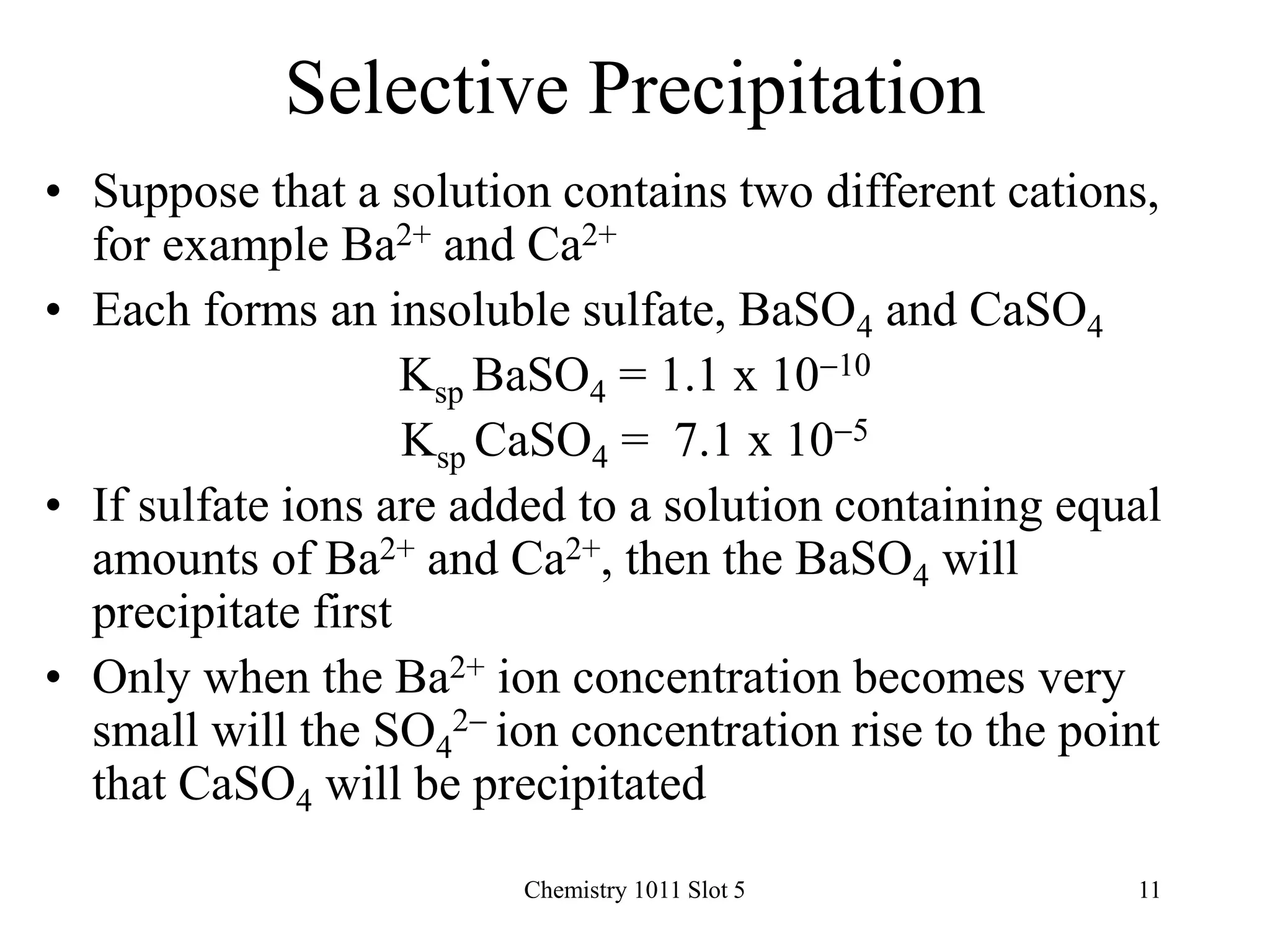
The document covers solubility equilibrium, including how to write expressions for solubility product constants (ksp), calculate ion concentrations and predict precipitate formation. Key concepts discussed include the formation of precipitates, Le Chatelier’s principle, and the common ion effect on solubility. Various examples demonstrate calculations related to ksp, determining precipitate formation, and effects of common ions on the solubility of salts.


![Chemistry 1011 Slot 5 4
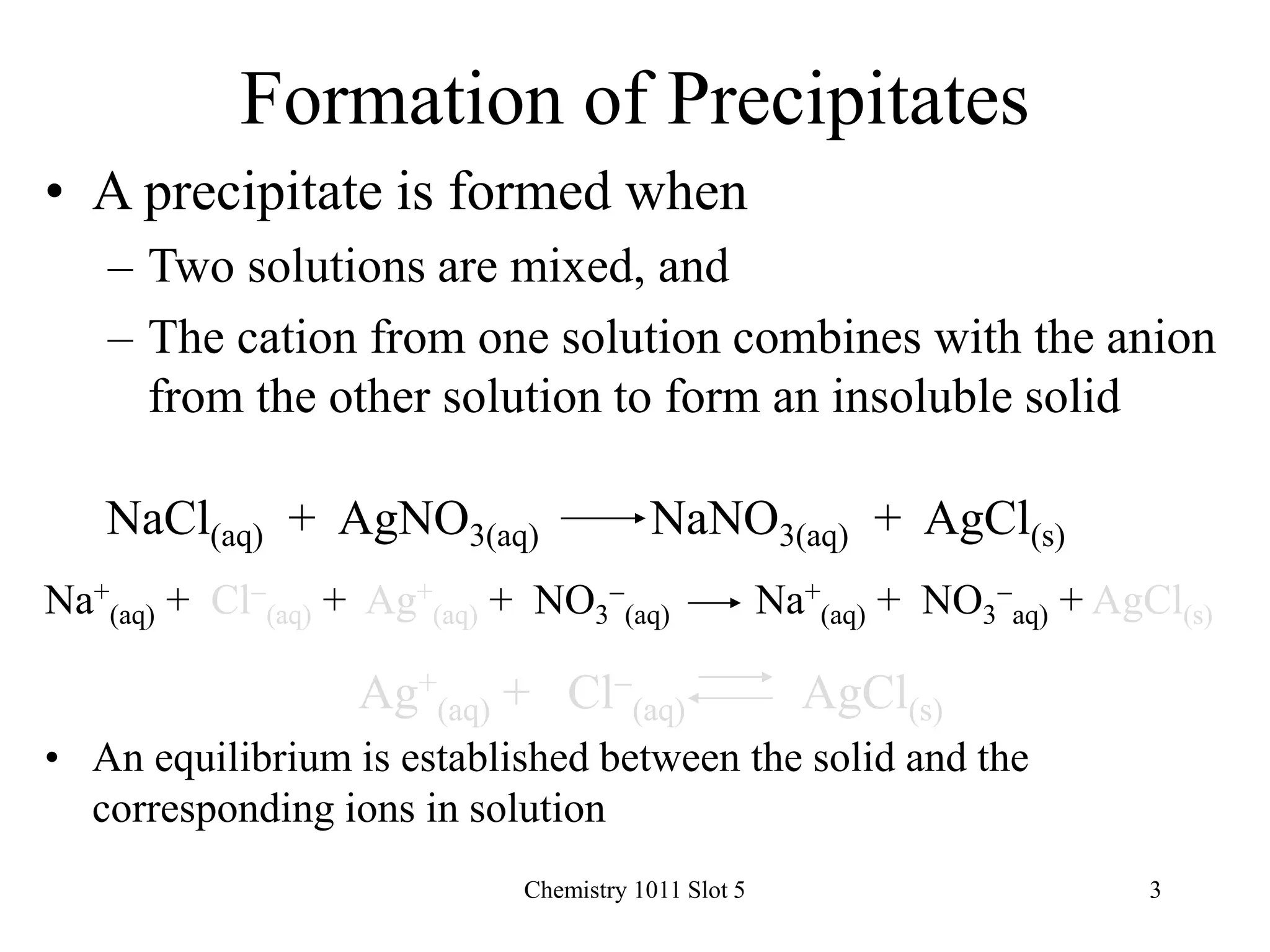
Solubility Equilibrium
AgCl(s) Ag+
(aq) + Cl-
(aq)
• An expression can be written for the equilibrium
constant:
Ksp = [Ag+]x[Cl-]
• [solid] does not appear in the equilibrium
constant expression
• Ksp is known as the solubility product constant
• Solubility product data are normally measured at
25oC](https://image.slidesharecdn.com/chemistry1011161-240719163502-156ac850/75/Solubility-and-solubility-product-examples-ppt-4-2048.jpg)
![Chemistry 1011 Slot 5 5
Determining Ion Concentrations
Ag3PO4(s) 3Ag+
(aq) + PO4
3-
(aq)
Ksp = [Ag+]3x[PO4
3-] = 1 x 10-16
PbCl2(s) Pb2+
(aq) + 2Cl-
(aq)
Ksp = [Pb2+]x[Cl-]2 = 1.7 x 10-5
Q: Calculate [Pb2+] and [Cl-] in a solution of PbCl2 at 25oC
[Cl-] = 2 x [Pb2+]
Ksp = [Pb2+] x [2Pb2+]2 = 1.7 x 10-5
Ksp = 4 [Pb2+]3 = 1.7 x 10-5
[Pb2+] = 1.6 x 10-2 mol/L [Cl-] = 3.2 x 10-2 mol/L](https://image.slidesharecdn.com/chemistry1011161-240719163502-156ac850/75/Solubility-and-solubility-product-examples-ppt-5-2048.jpg)
![Chemistry 1011 Slot 5 6
Calculating Ksp
• The solubility of a salt can be determined by experiment
• Ksp for the salt can be determined from these results
• Q: The solubility of magnesium hydroxide is found to be 8.4 x 10-4
g/100cm3 at 25oC. Find Ksp
Mg(OH)2(s) Mg2+
(aq) + 2OH-
(aq)
Ksp = [Mg2+]x[OH-]2 = ??
Solubility = 8.4 x 10-4 g/100cm3 at 18oC
Solubility = (8.4 x 10-4)g x 1000cm3/L = 1.44 x 10-4 mol/L
58.3 g/mol 100cm3
[Mg2+] = 1.44 x 10-4 mol/L; [OH-] = 2.88 x 10-4 mol/L
Ksp = [Mg2+]x[OH-]2 = 1.2 x 10-11](https://image.slidesharecdn.com/chemistry1011161-240719163502-156ac850/75/Solubility-and-solubility-product-examples-ppt-6-2048.jpg)

![Chemistry 1011 Slot 5 8
Determining Precipitate Formation
Q: Will a precipitate form when 5.0mL of 1.0 x 10-3 mol/L silver
nitrate is added to 5.0mL of 1.0 x 10-5 mol/L potassium chromate?
Ksp Ag2CrO4 = 1.0 x 10-12
2AgNO3(aq) + K2CrO4(aq) 2KNO3(aq) + Ag2CrO4(s)
2Ag+
(aq) + CrO4
2-
(aq) Ag2CrO4(s)
Ksp = [Ag+]2 x [CrO4
2-] = 1.0 x 10-12
[Ag+] = 5.0 x 10-4 mol/L
[CrO4
2-] = 5.0 x 10-6 mol/L
Ion Product, P = [Ag+]2 x [CrO4
2-] = (5.0 x 10-4 )2 x (5.0 x 10-6 )
P = 1.25 x 10-12
P > Ksp A precipitate will form](https://image.slidesharecdn.com/chemistry1011161-240719163502-156ac850/75/Solubility-and-solubility-product-examples-ppt-8-2048.jpg)

![Chemistry 1011 Slot 5 10
Determining Precipitate Formation
• 1.0 mL of 1.0 mol/L barium chloride is added to 10.0 mL of a solution containing a small
amount of magnesium sulfate. Determine the minimum concentration of magnesium sulfate
that will cause a precipitate to form. Ksp BaSO4 = 1.1 x 10-10
BaCl2(aq) + MgSO4(aq) MgCl2(aq) + BaSO4(s)
Ba2+
(aq) + SO4
2-
(aq) BaSO4(s)
Ksp = [Ba2+]x[SO4
2-] = 1.1 x 10-10
[Ba2+] = 1.0 x 10-3L x 1.0 mol/L 1.00 x 10-2L = 1.0 x 10-1 mol/L
[SO4
2-] = x mol/L
Ksp = [Ba2+]x[SO4
2-] = (1.0 x 10-1 ) x (x) = 1.1 x 10-10
x = [SO4
2-] = minimum [MgSO4] =1.1 x 10-10 mol/L](https://image.slidesharecdn.com/chemistry1011161-240719163502-156ac850/75/Solubility-and-solubility-product-examples-ppt-10-2048.jpg)
![Chemistry 1011 Slot 5 12
Determining Solubility
• The solubility, s, of a salt can be determined from Ksp data
Q: Determine the solubility of lead chloride in water at 25oC. Ksp =
1.7 x 10-5
Let solubility of lead chloride = s mol/L
• For every mole of PbCl2 that dissolves,
1 mole of Pb2+
(aq) and 2 moles of Cl-
(aq) are formed
PbCl2(s) Pb2+
(aq) + 2Cl-
(aq)
[Pb2+] = s mol/L
[Cl-] = 2 x [Pb2+] = 2s mol/L
Ksp = [Pb2+]x[Cl-]2 = (s) x(2s)2 = 1.7 x 10-5
4s3 = 1.7 x 10-5
s = 1.6 x 10-2 mol/L (Can also be expressed in grams/Litre)](https://image.slidesharecdn.com/chemistry1011161-240719163502-156ac850/75/Solubility-and-solubility-product-examples-ppt-12-2048.jpg)
![Chemistry 1011 Slot 5 13
The Common Ion Effect
• The presence of a common ion will reduce the solubility
of an ionic salt (Le Chatelier)
• If a common ion is added to a saturated solution of a salt,
then the salt will be precipitated (Le Chatelier)
For example, CaCO3 is less soluble in a solution
containing CO3
2- ions than in pure water
CaCO3(s) Ca2+
(aq) + CO3
2-
(aq)
Ksp = [Ca2+] x [CO3
2-] = 4.9 x 10-9](https://image.slidesharecdn.com/chemistry1011161-240719163502-156ac850/75/Solubility-and-solubility-product-examples-ppt-13-2048.jpg)
![Chemistry 1011 Slot 5 14
The Common Ion Effect
CaCO3(s) Ca2+
(aq) + CO3
2-
(aq)
Ksp = [Ca2+] x [CO3
2-] = 4.9 x 10-9
• Solubility of CaCO3 = [Ca2+]
• In pure water
[Ca2+] = [CO3
2-] = (4.9 x 10-9) = 7.0 x 10-5 mol/L
Solubility of CaCO3 in 1.0 x 10-1 sodium carbonate solution = ???
• [CO3
2-] = 1.0 x 10-1 mol/L (ignore CO3
2- from CaCO3 )
• [Ca2+] = Ksp = 4.9 x 10-9 = 4.9 x 10-8 mol/L
[CO3
2-] 1.0 x 10-1](https://image.slidesharecdn.com/chemistry1011161-240719163502-156ac850/75/Solubility-and-solubility-product-examples-ppt-14-2048.jpg)
![Chemistry 1011 Slot 5 15
One More Example
• Ksp for manganese II hydroxide is 1.2 x 10-11
• Solid sodium hydroxide is added slowly to a 0.10 mol/L
solution of manganese II chloride. What will be the pH when
a precipitate forms?
Mn(OH)2(s) Mn2+
(aq) + 2OH-
(aq)
Ksp = [Mn2+] x [OH-]2 = 1.2 x 10-11
• [Mn2+] = 0.10 mol/L
• [OH-] = Ksp [Mn2+] = 1.2 x 10-11 0.10
= 1.1 x 10-5 mol/L
• pH = 9.0](https://image.slidesharecdn.com/chemistry1011161-240719163502-156ac850/75/Solubility-and-solubility-product-examples-ppt-15-2048.jpg)