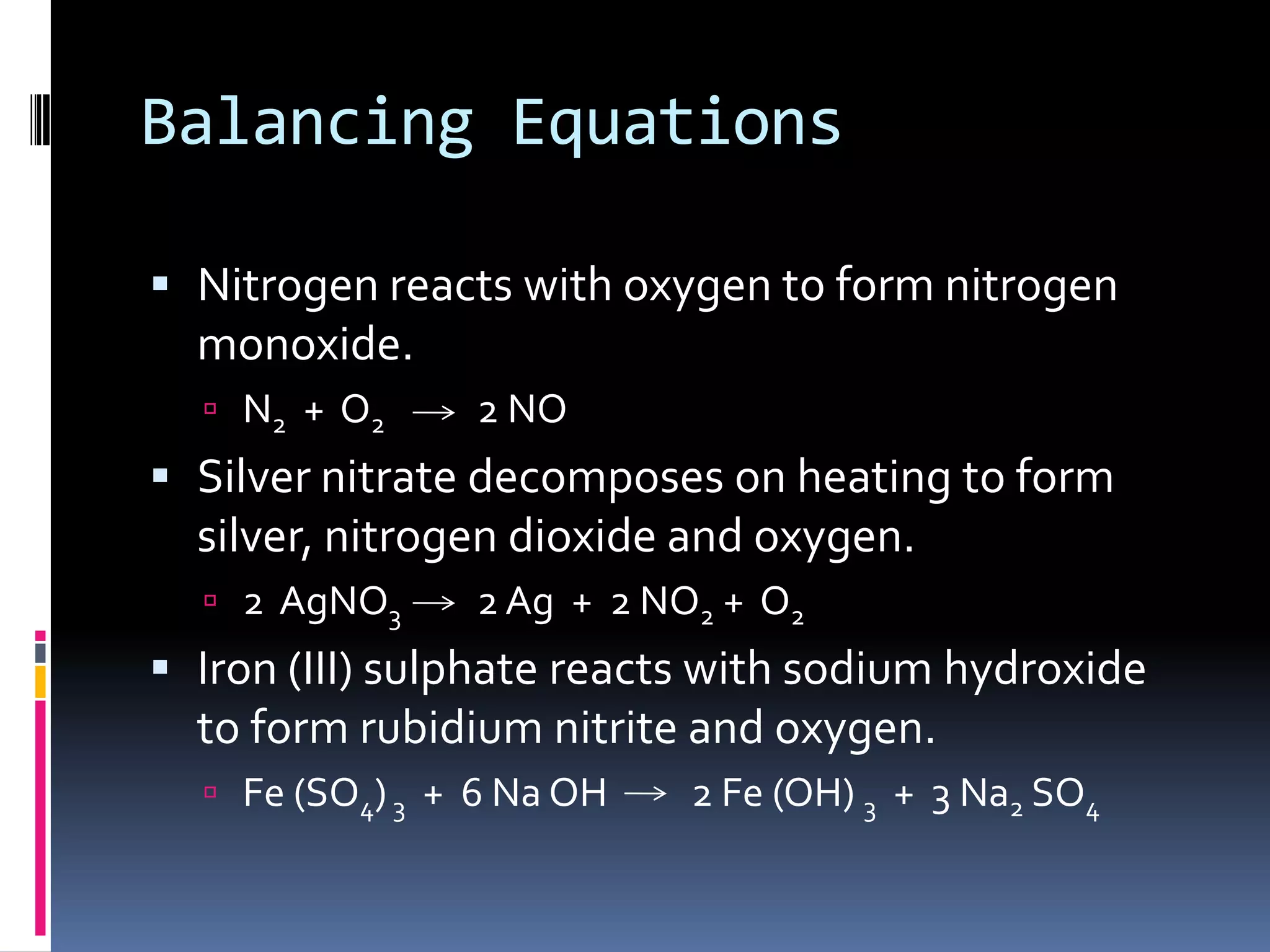
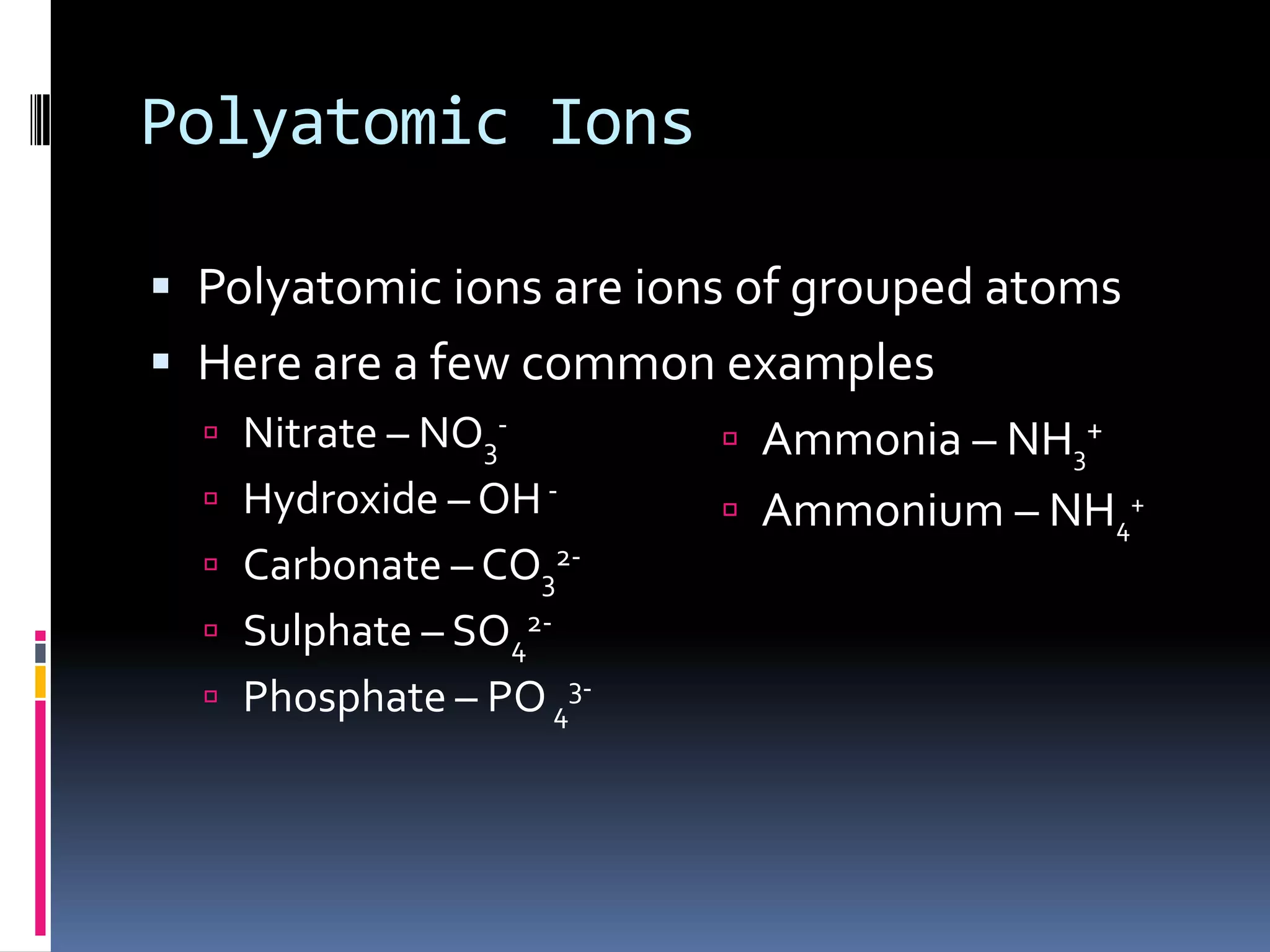
This document discusses several chemistry concepts including isotopes, ions, and bonding. It defines isotopes as atoms of the same element with different numbers of neutrons but the same number of protons. Metals form cations by losing electrons while non-metals form anions by gaining electrons. There are two main types of bonding - ionic bonding between metals and non-metals formed by electrovalent bonds, and covalent bonding where non-metals share electrons. Chemical equations are used to represent chemical reactions and must be balanced.


![Cations and AnionsMetals from cations (positively charged ions) by losing electrons.Formation of cations : (Sodium) Na [Na] +Non-metals form anions (negatively charged ions) by gaining electrons.Formation of anions : (Oxygen) O [O] 2-](https://image.slidesharecdn.com/solubilityandequations-090717043610-phpapp01/75/Solubility-And-Equations-3-2048.jpg)

![Ionic bondingIonic bonding between metals and non-metals is formed by electrovalent bonds.Examples :Na ++ Cl-[Na]++ [Cl]-Na ClMg 2++ Cl -[Mg] 2++ 2[Cl]- Mg Cl2Notice that the ions of both elements are swapped to become the chemical formula of the compound.This also applies to covalent compounds.](https://image.slidesharecdn.com/solubilityandequations-090717043610-phpapp01/75/Solubility-And-Equations-6-2048.jpg)
![Covalent bondingNon-metals share electrons to create covalent bonding between one other.Examples :H-+ O2-[H]1+1=2 [O]6+2=8 H2OC4-+ H-[C]4+4=8 [H]1+1=2 CH4Although the formation of the chemical formula is the same as ionic compounds, the method of bonding is different.Both atoms gain electrons through sharing.](https://image.slidesharecdn.com/solubilityandequations-090717043610-phpapp01/75/Solubility-And-Equations-7-2048.jpg)