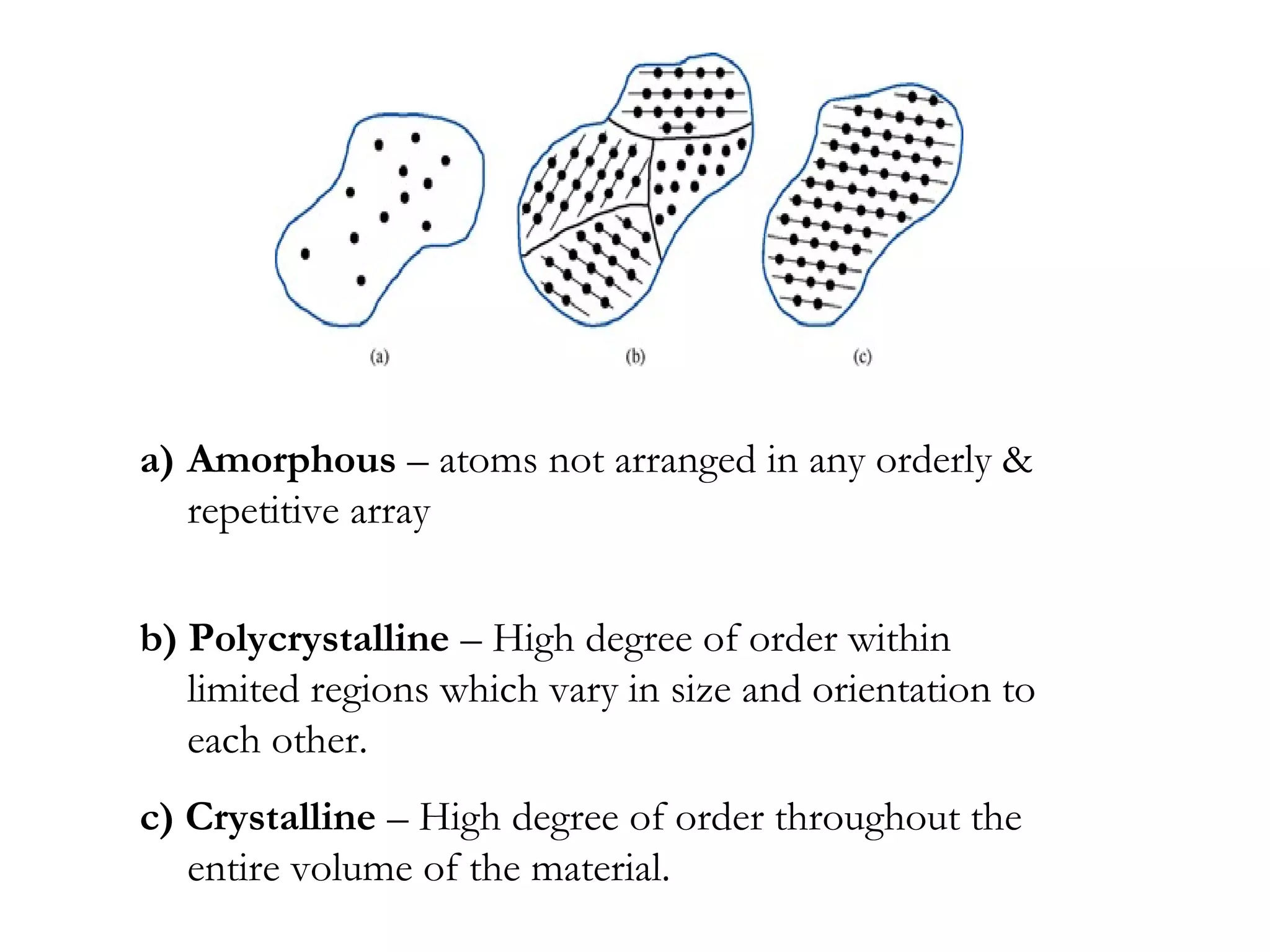
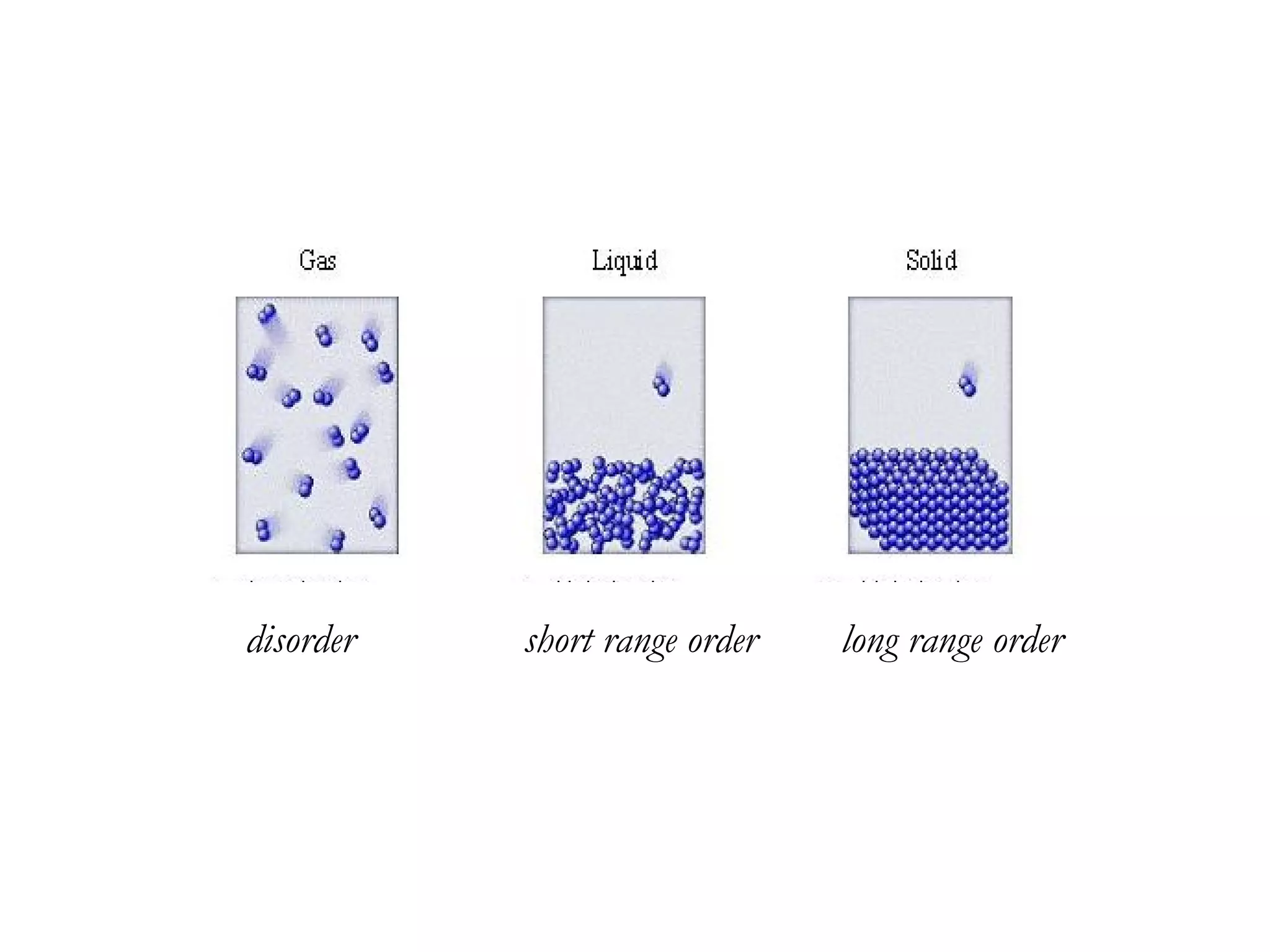
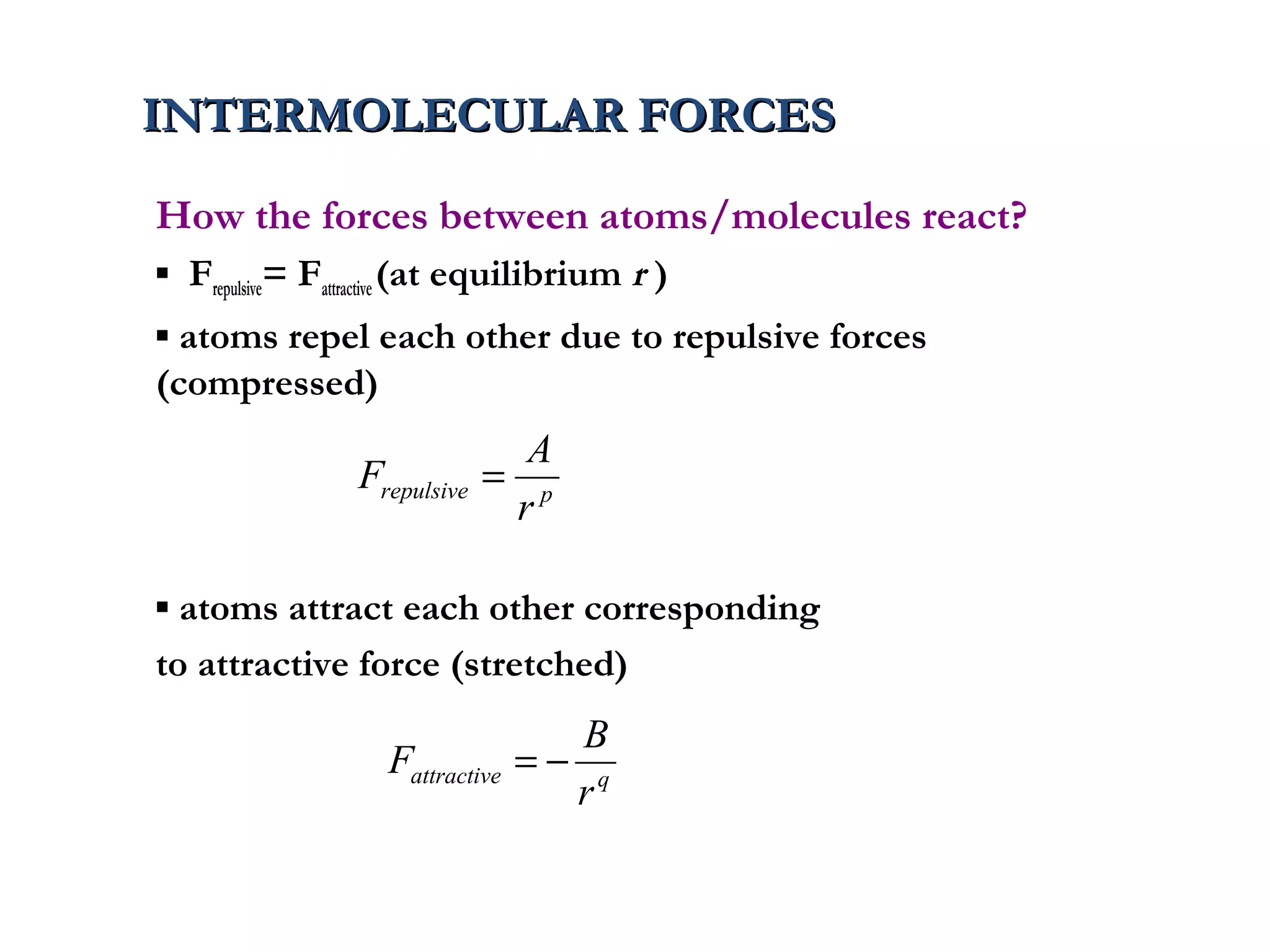
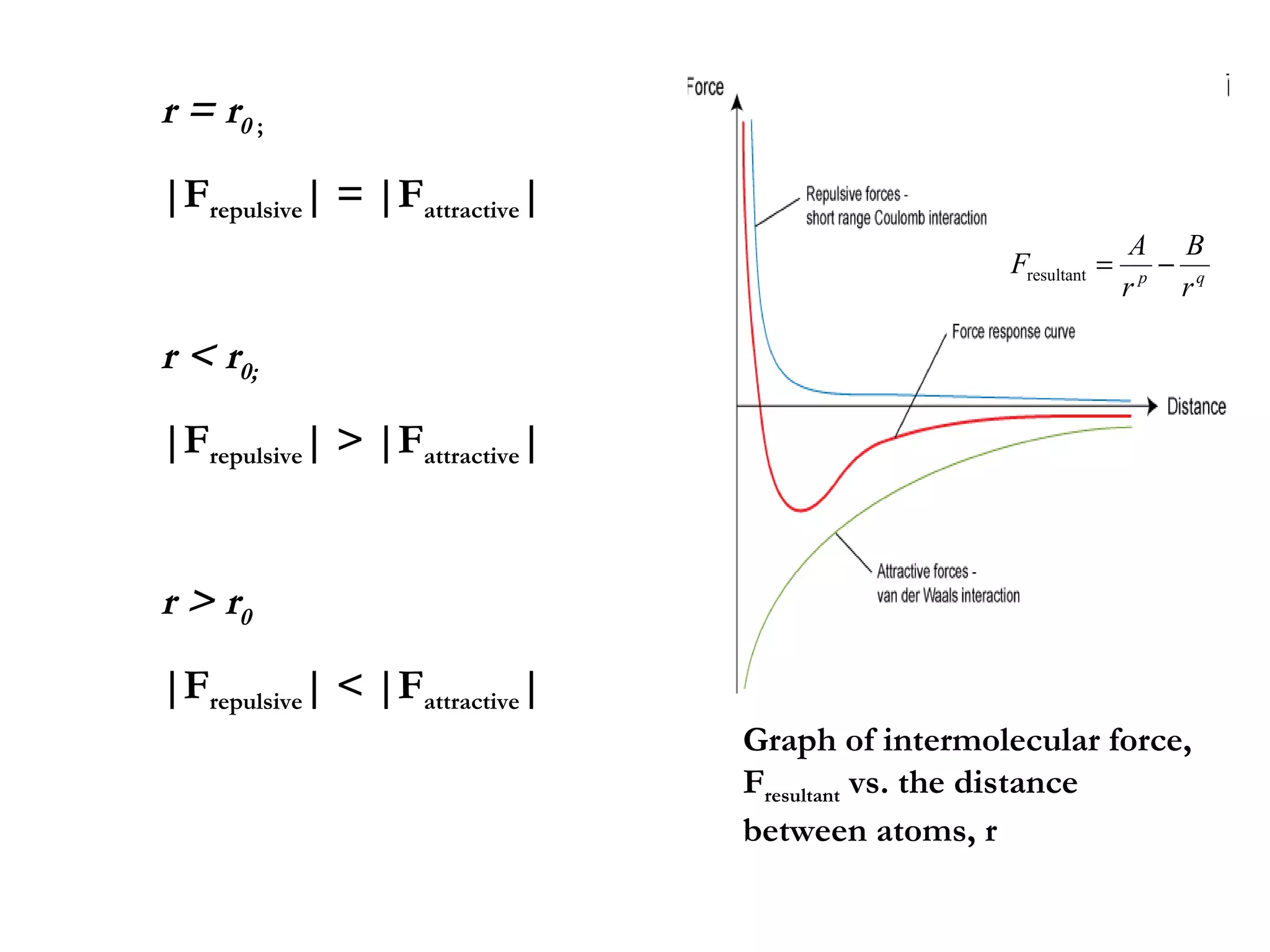
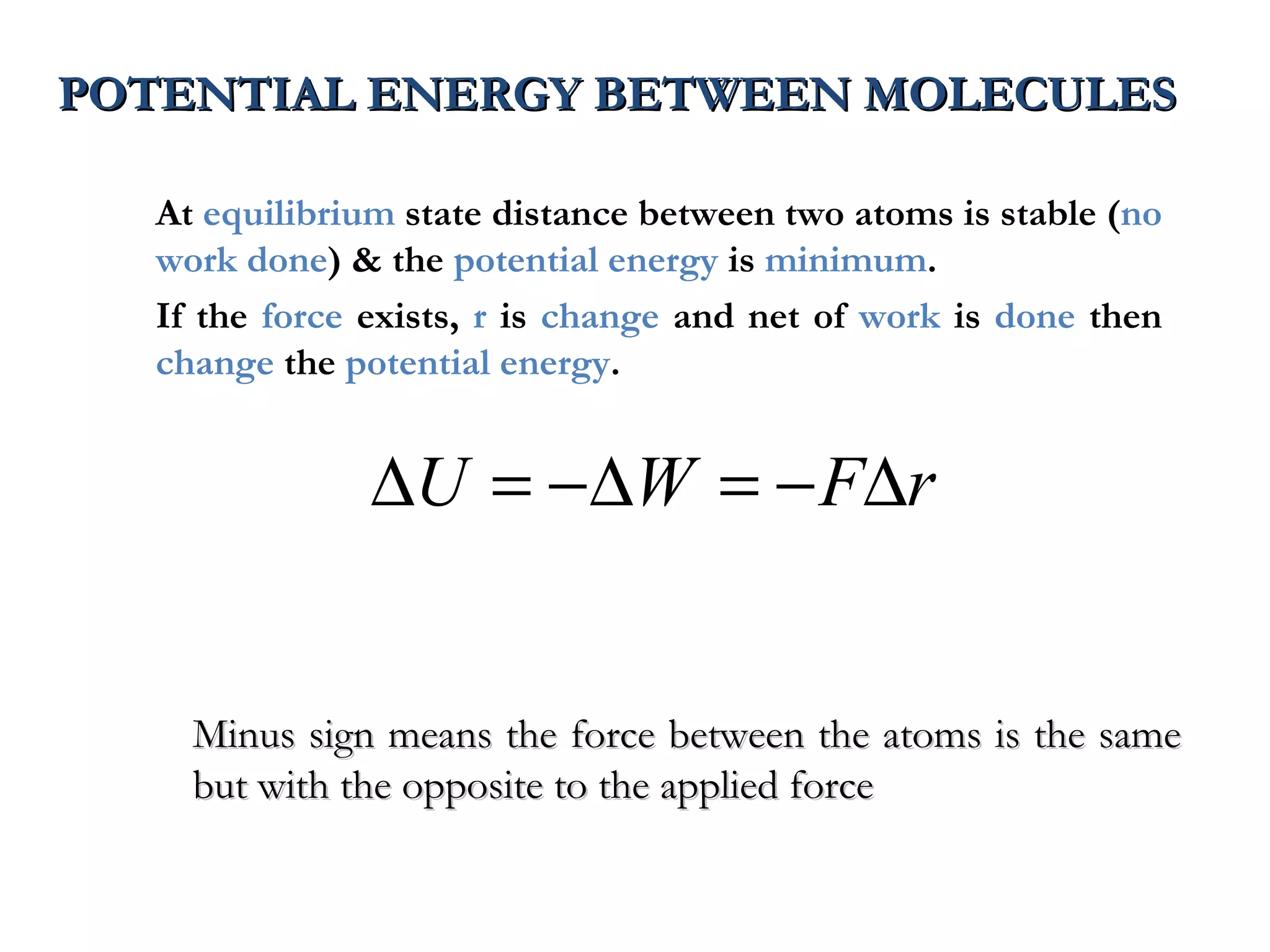
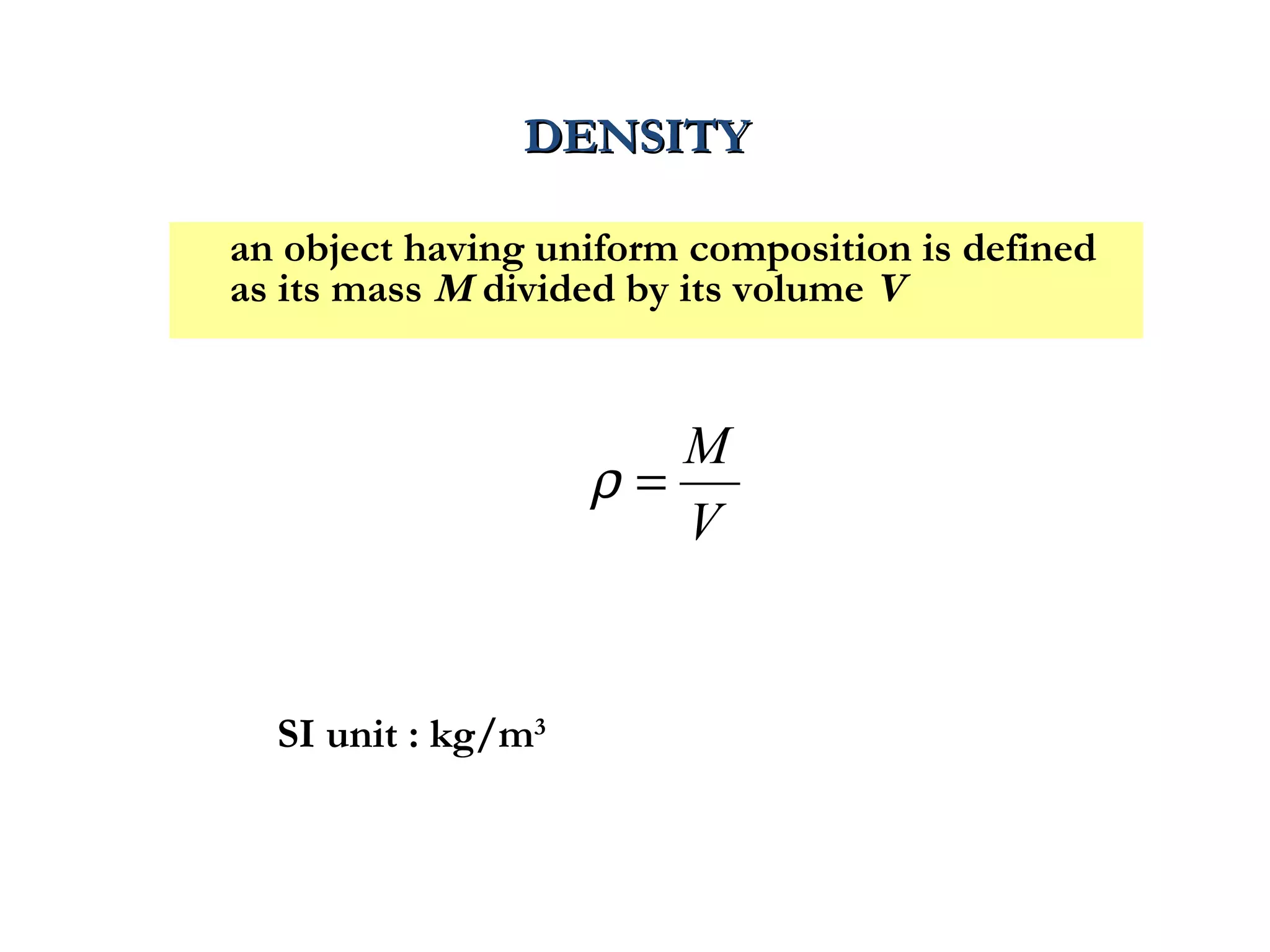
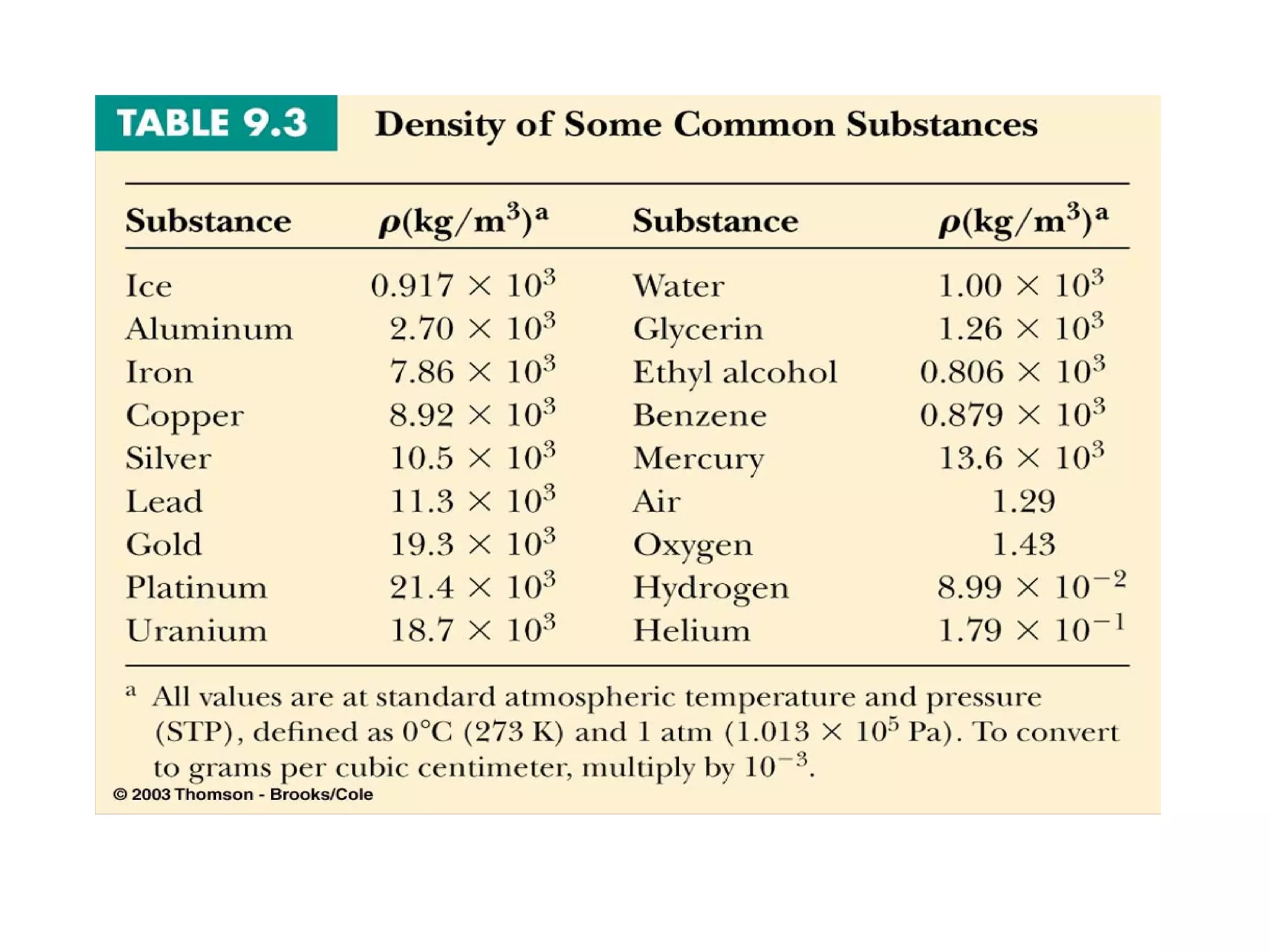
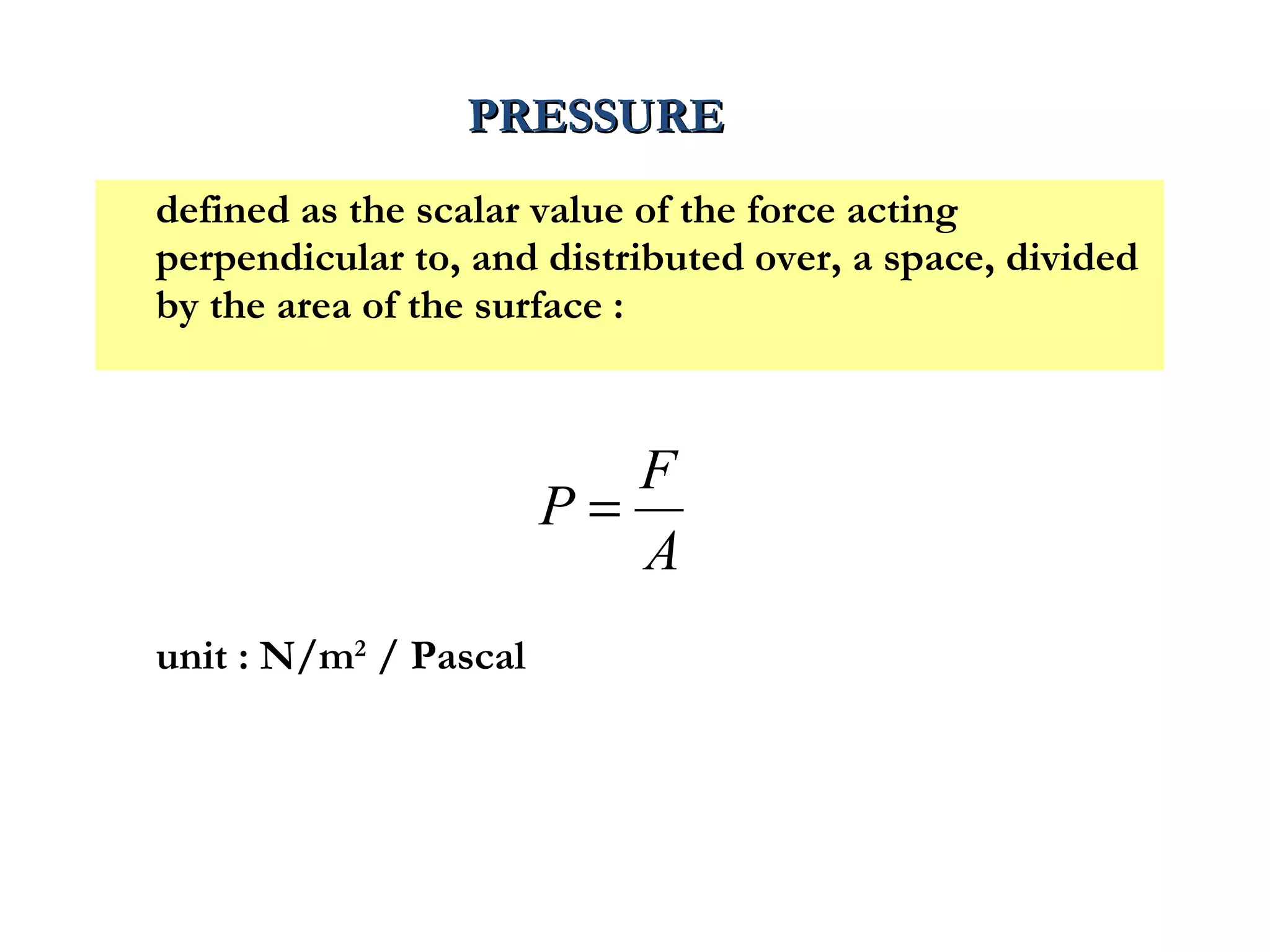
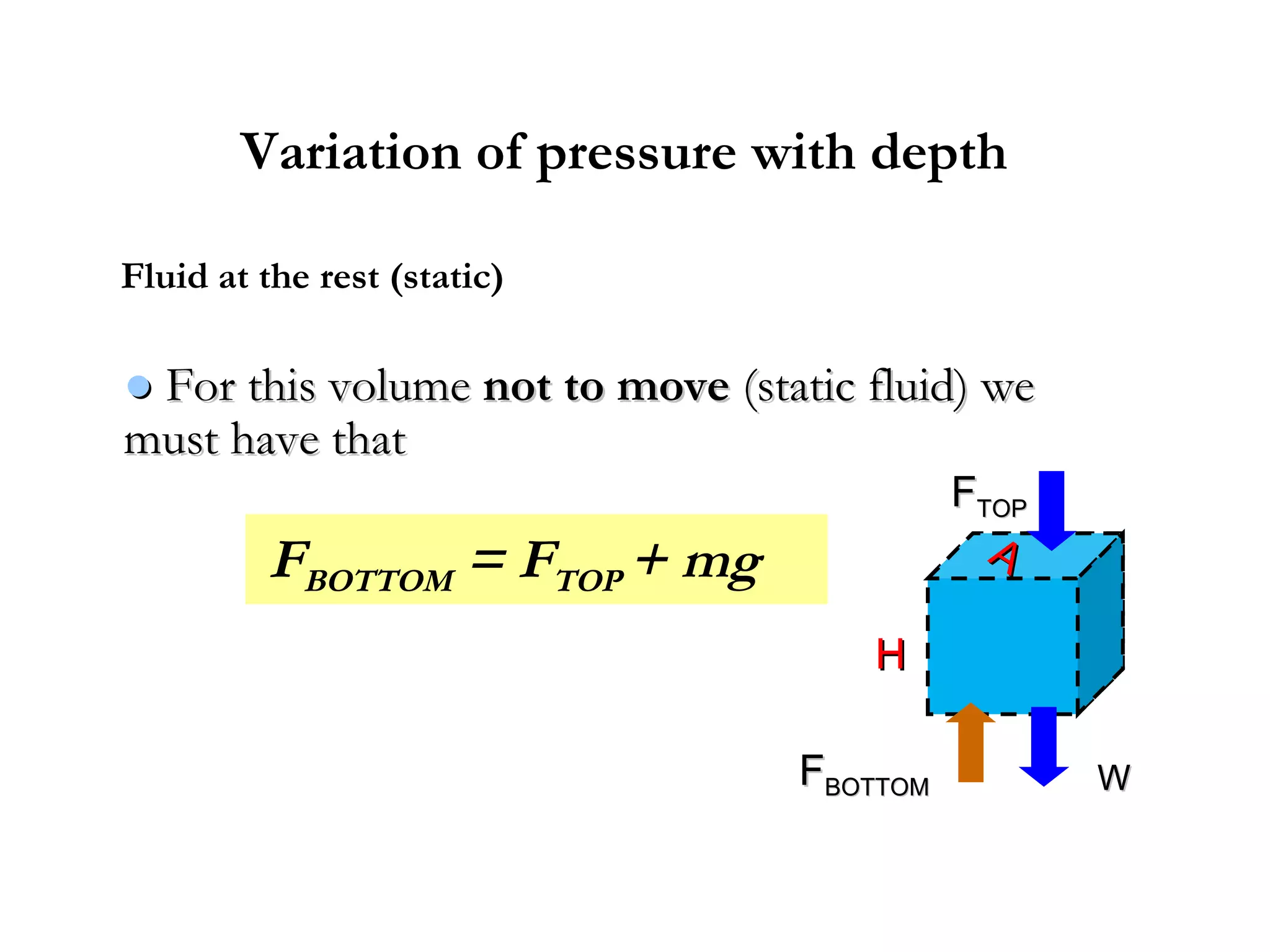
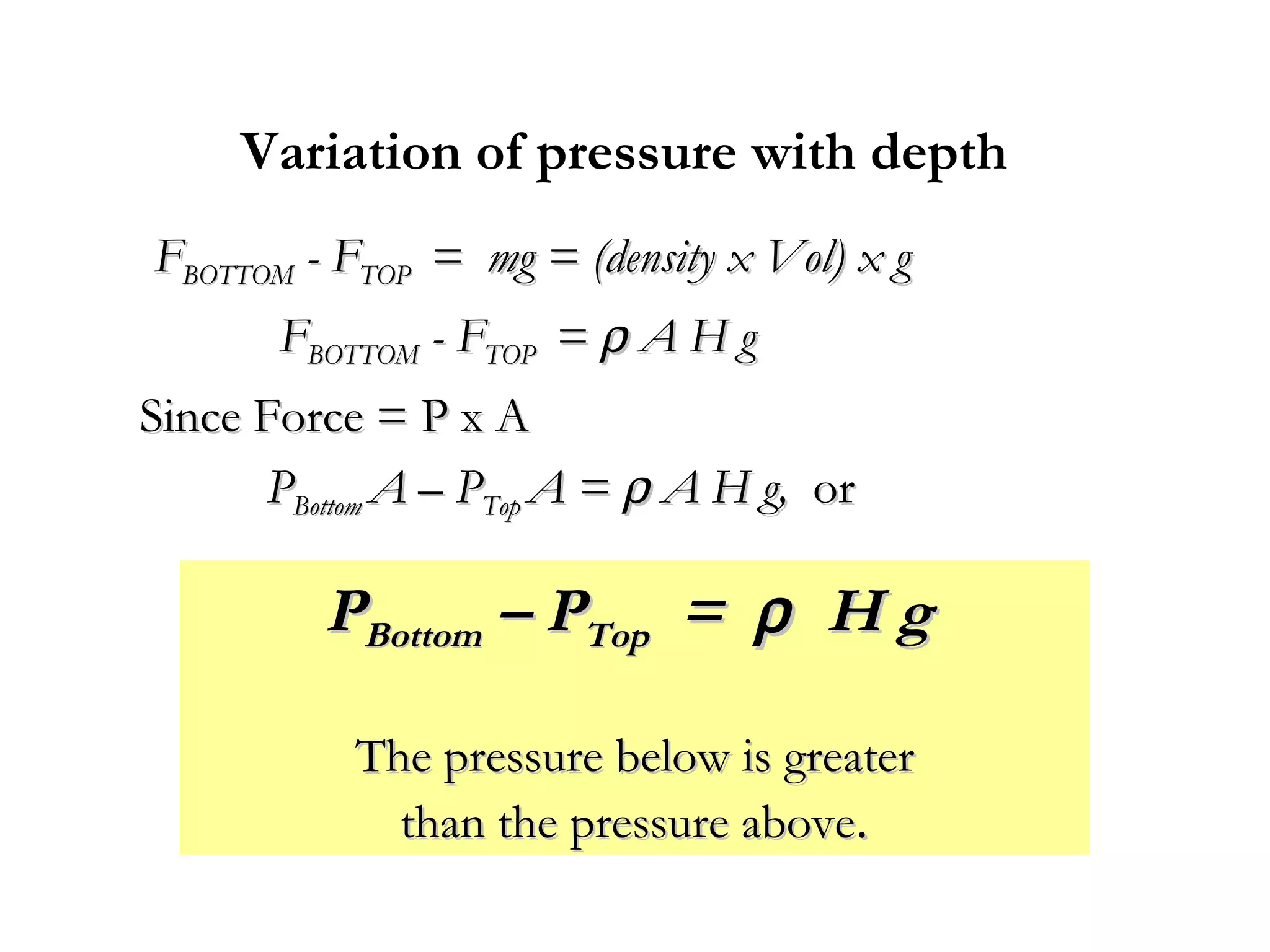
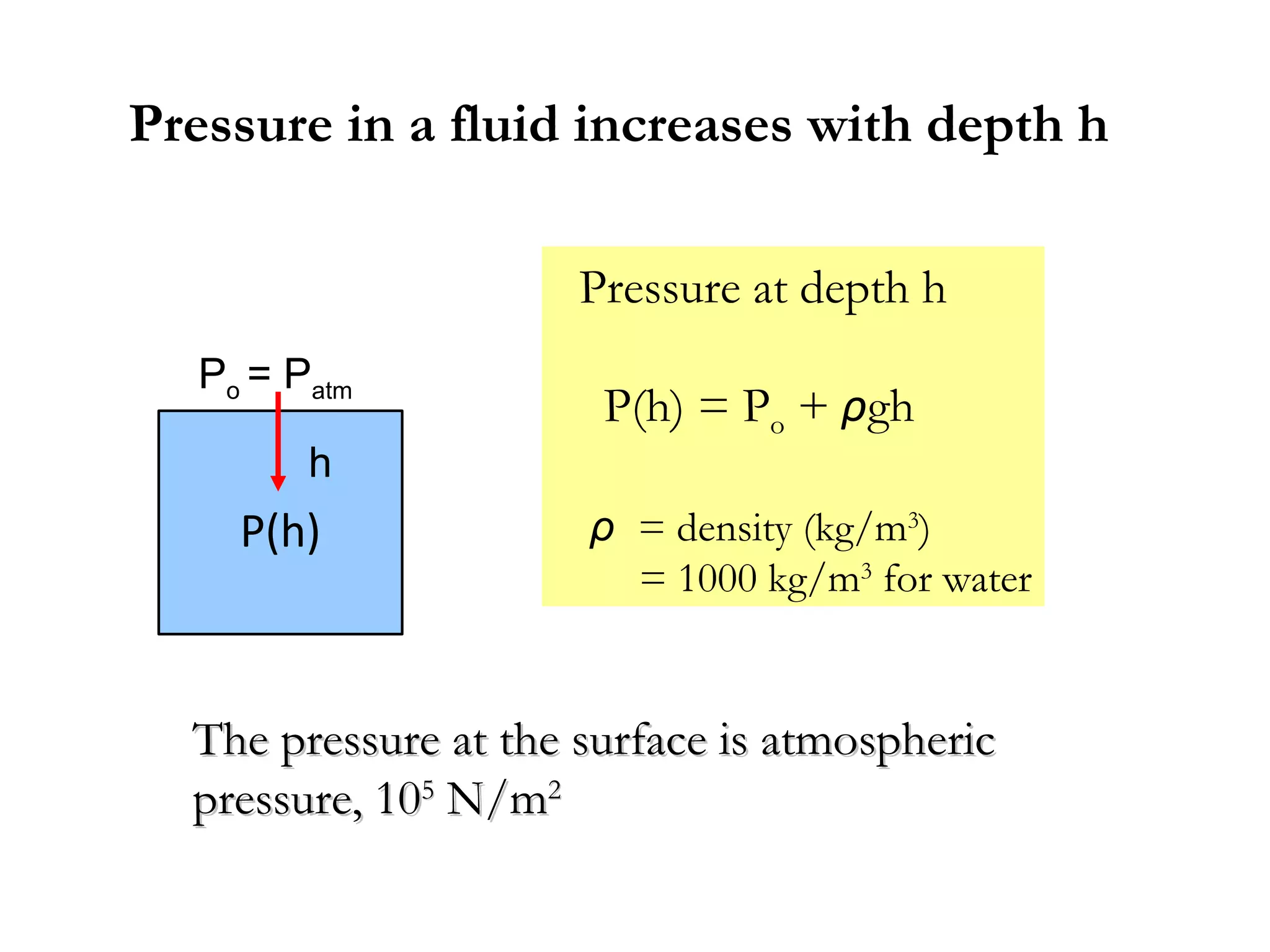
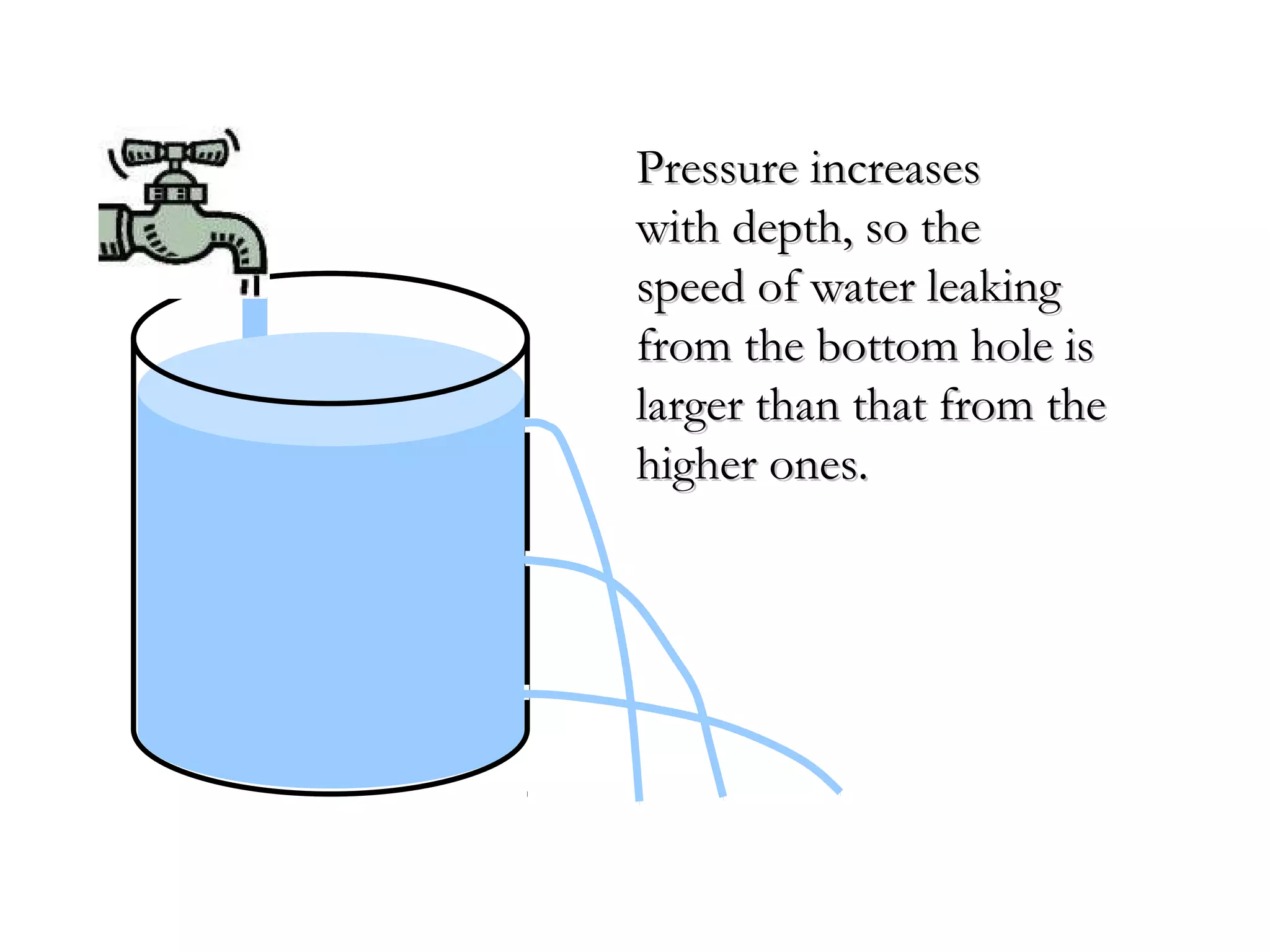
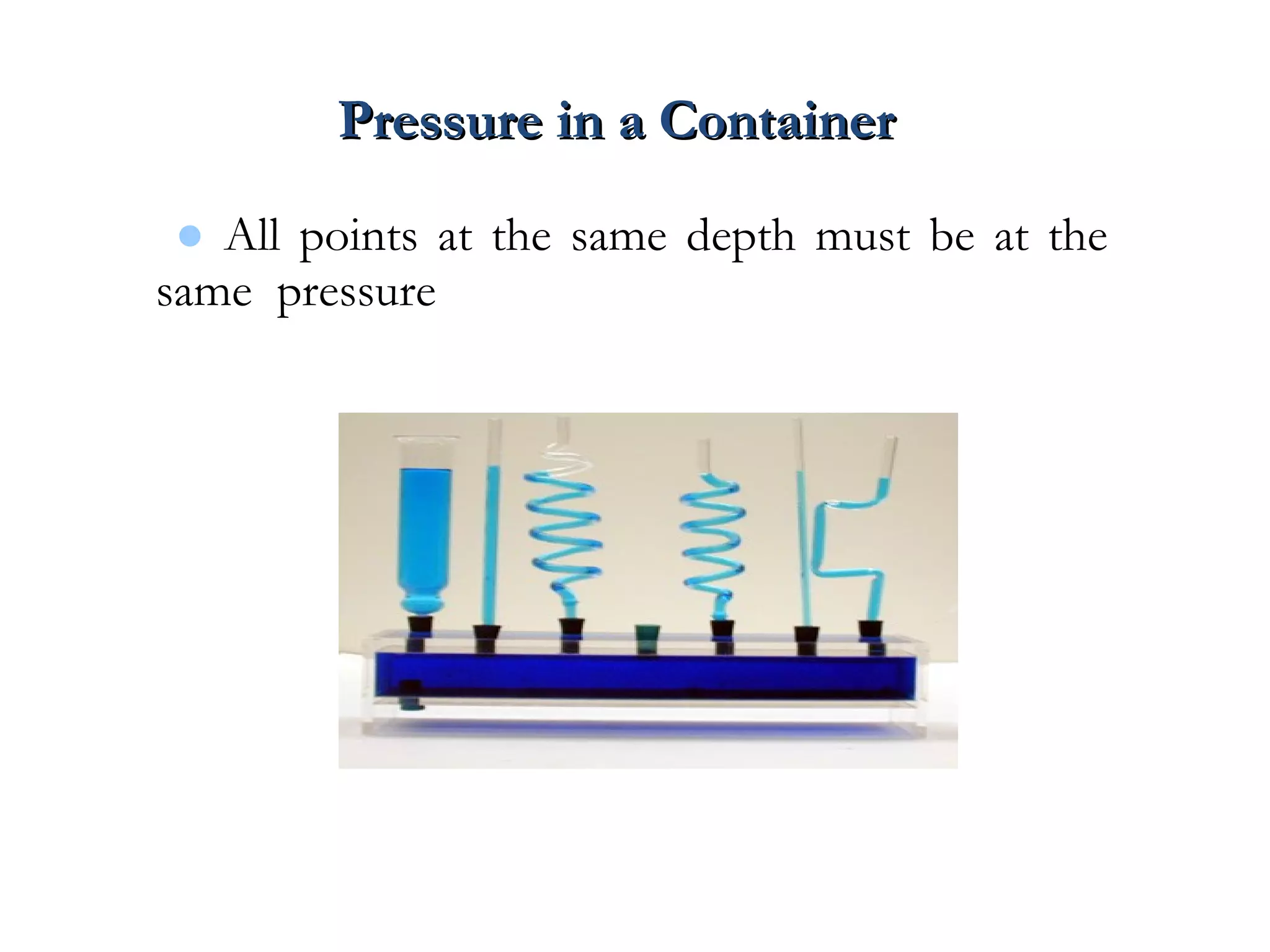
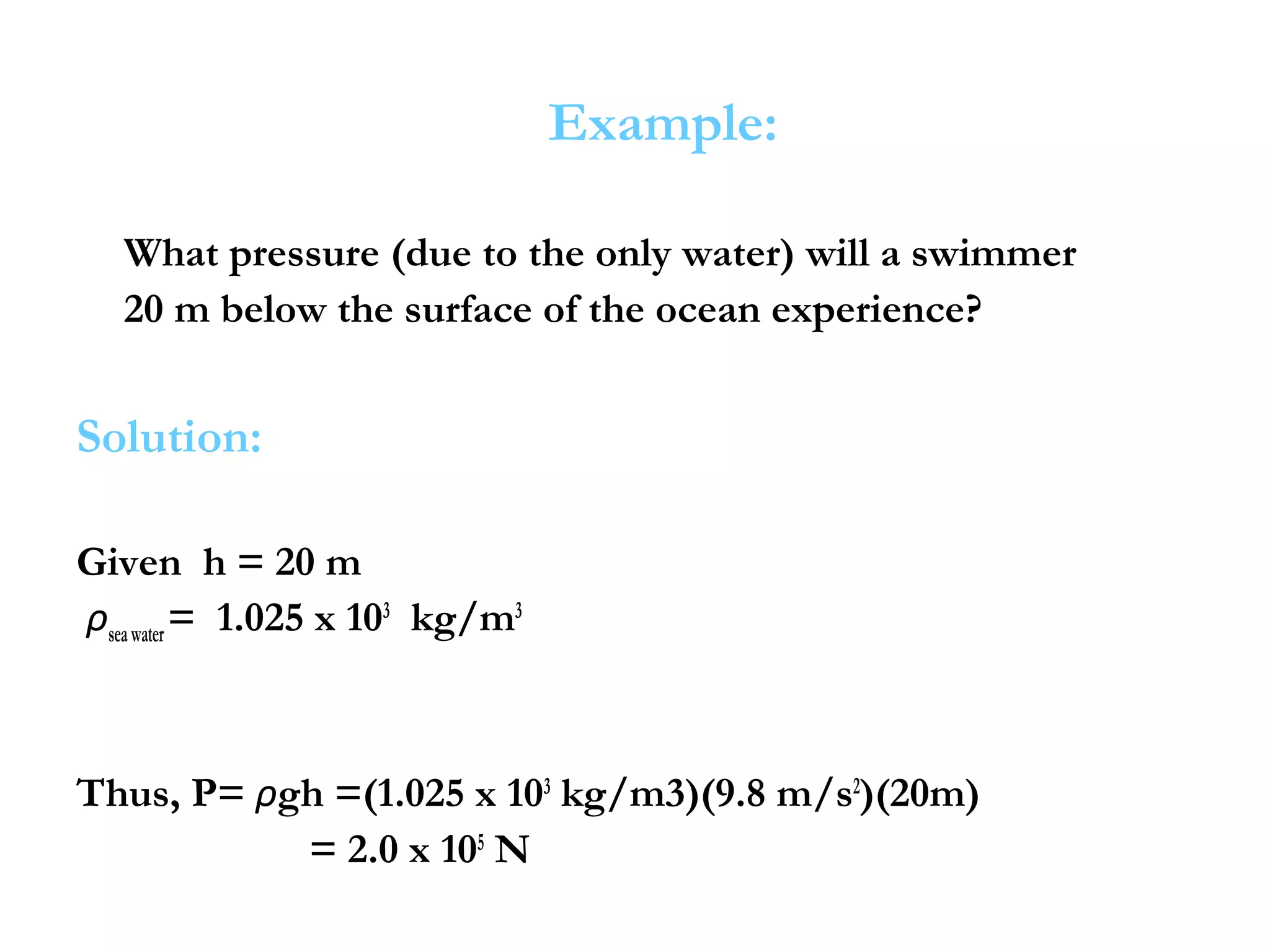
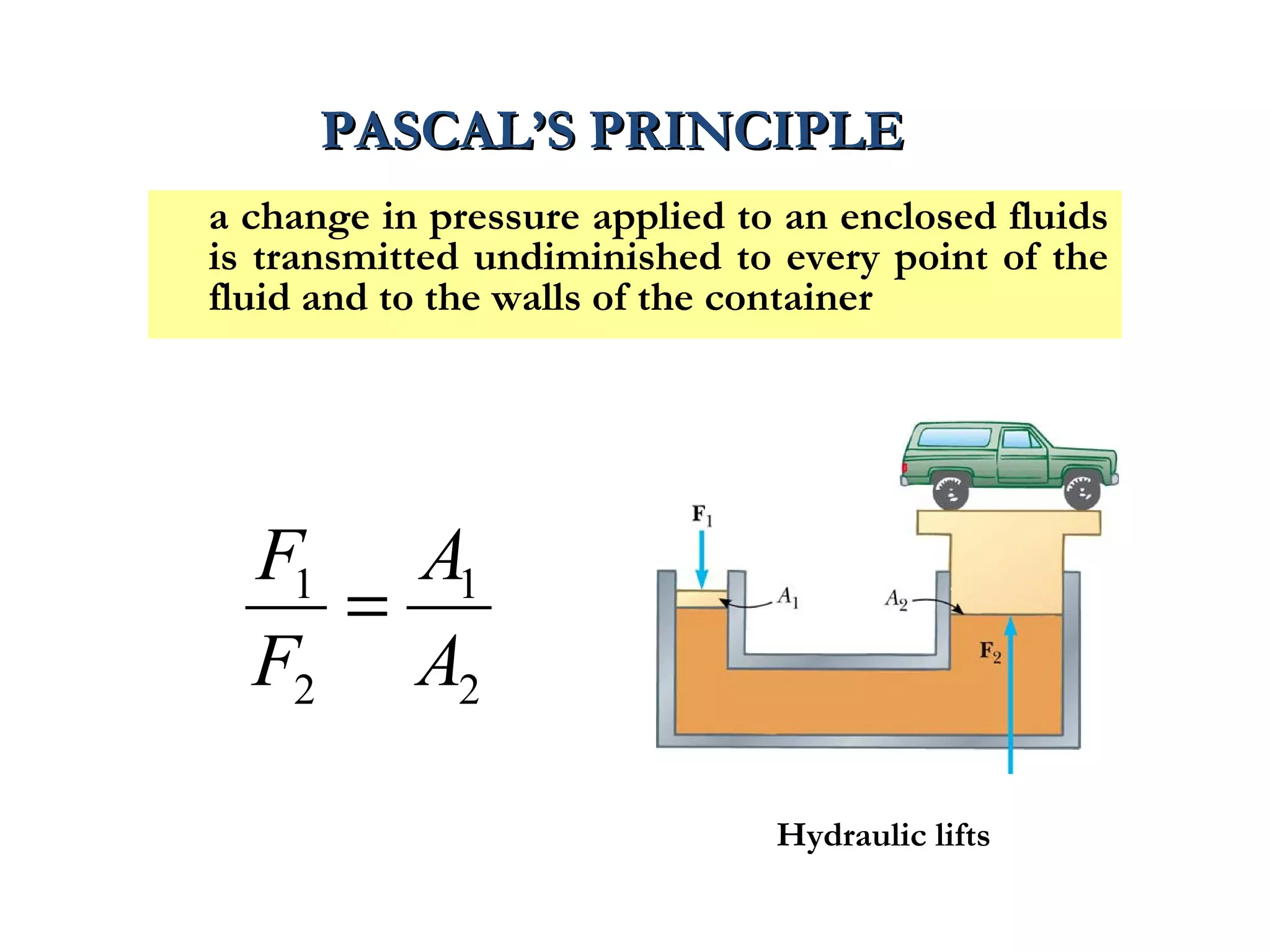
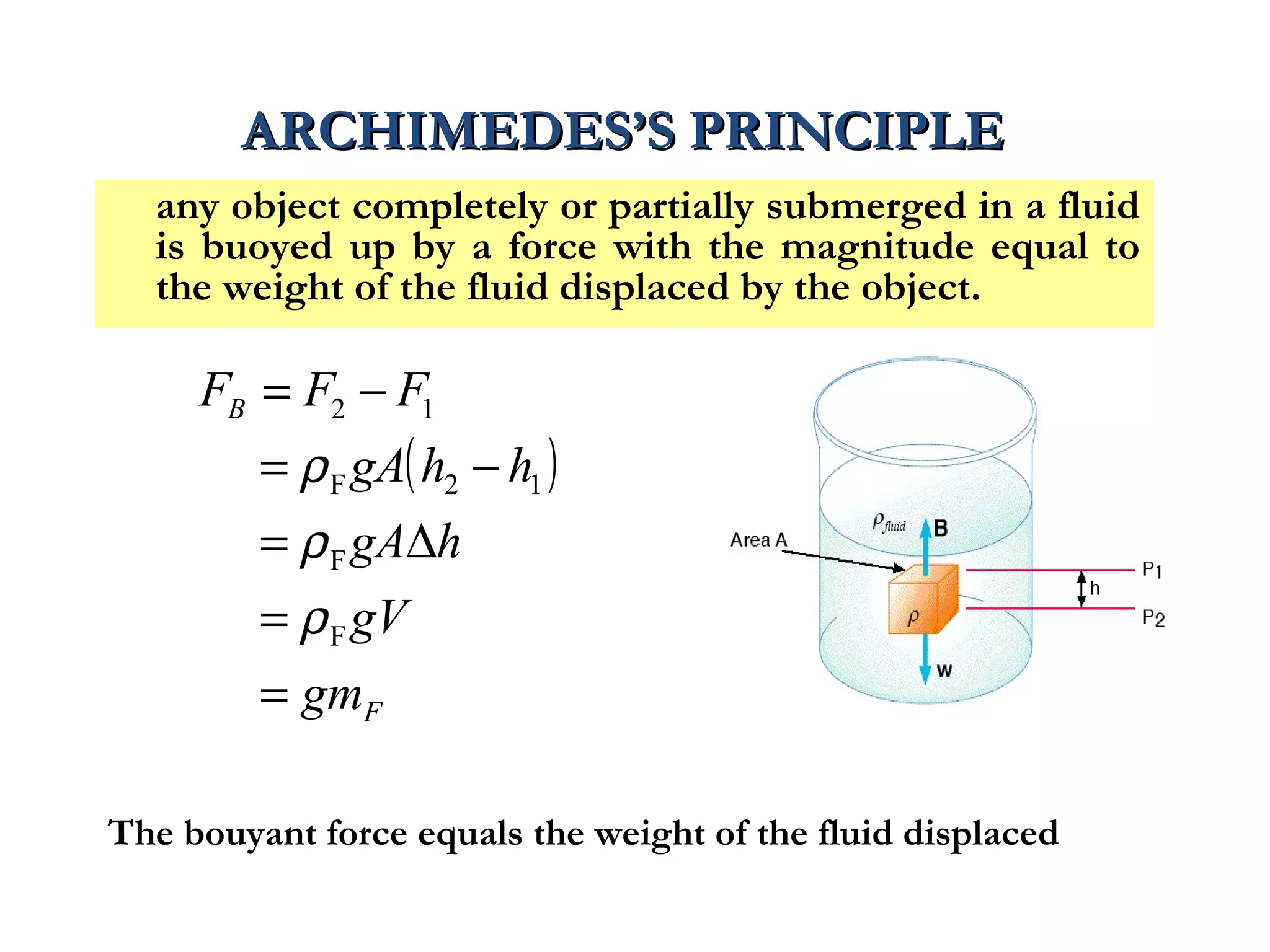
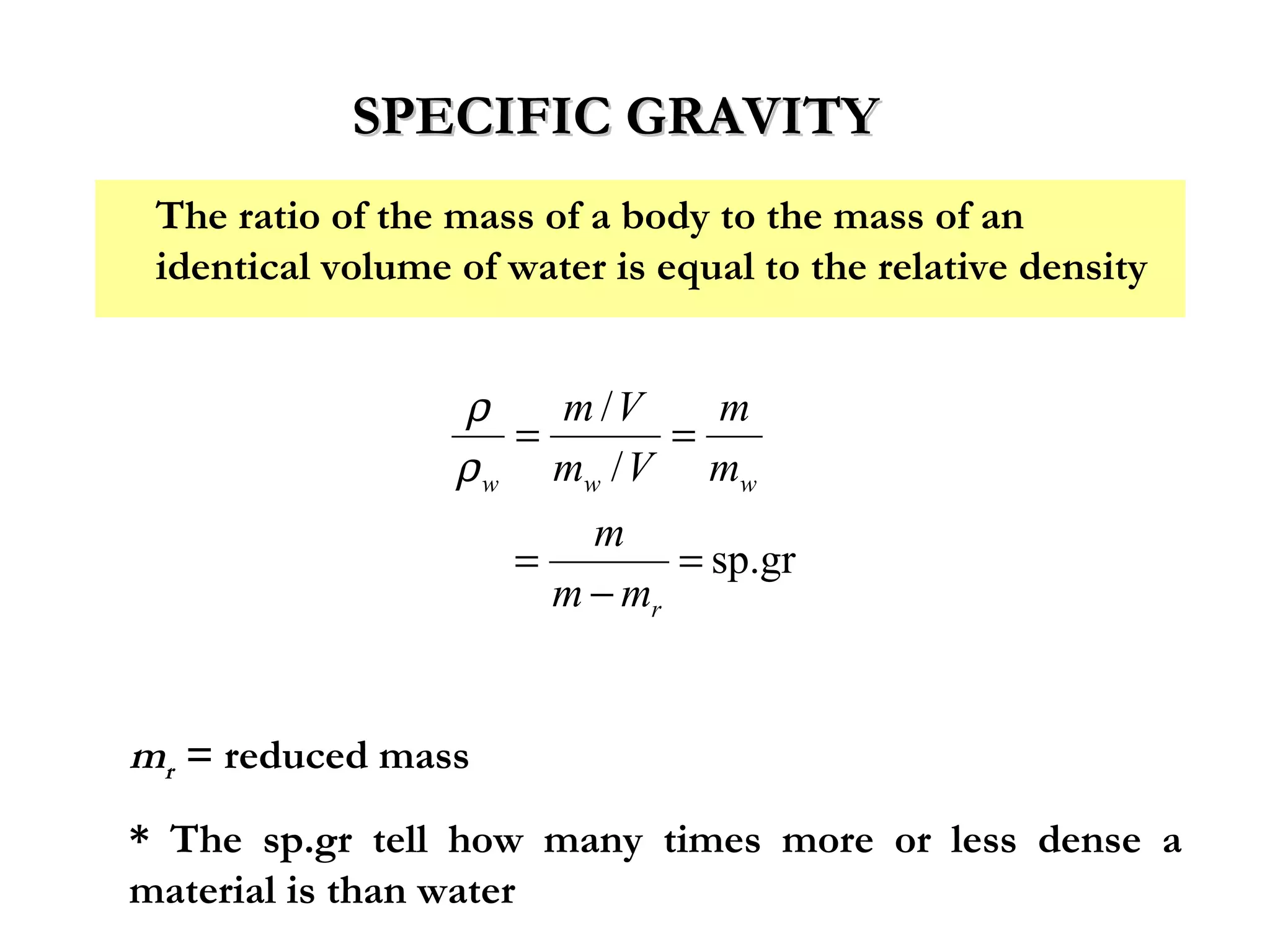
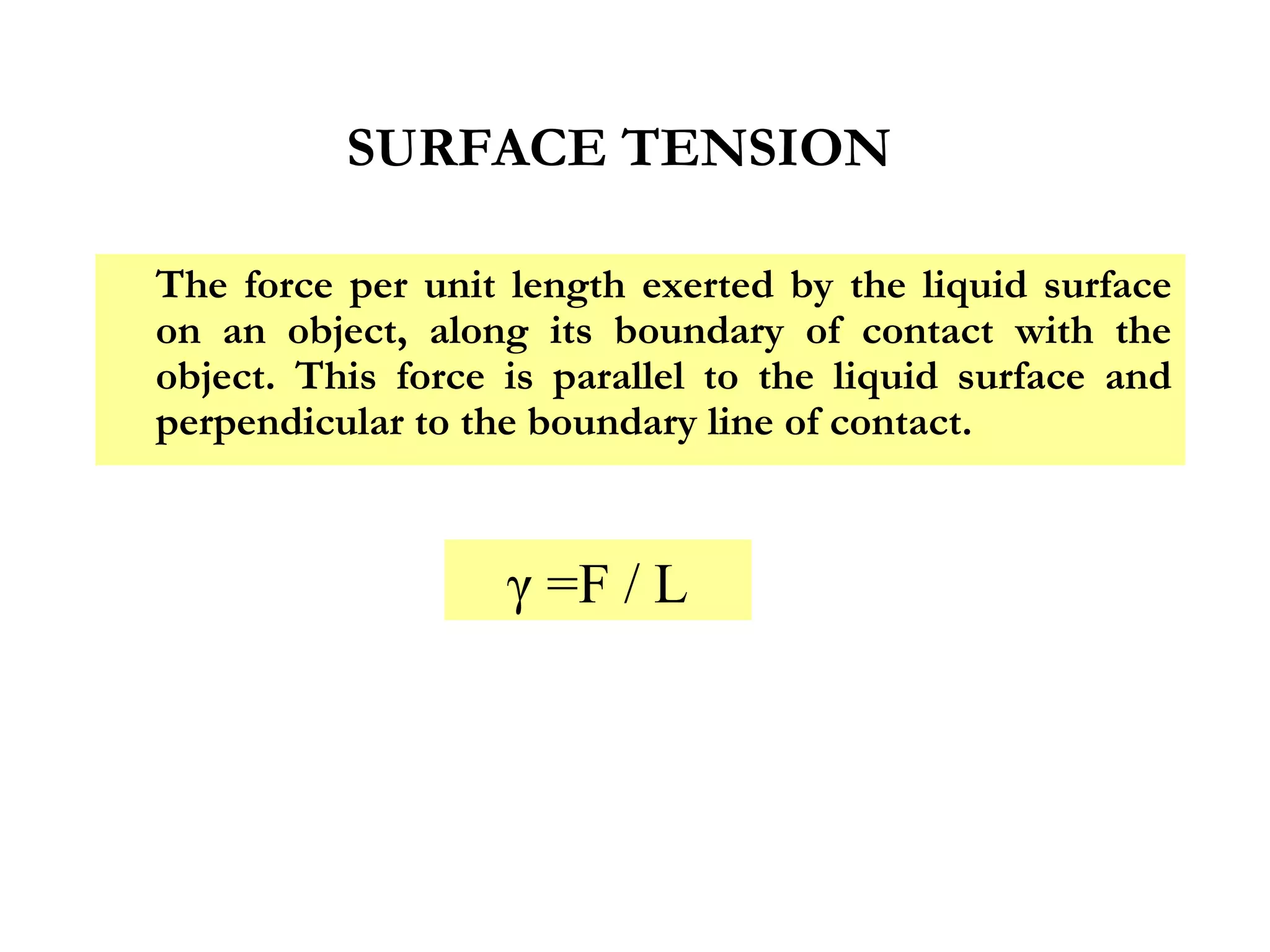
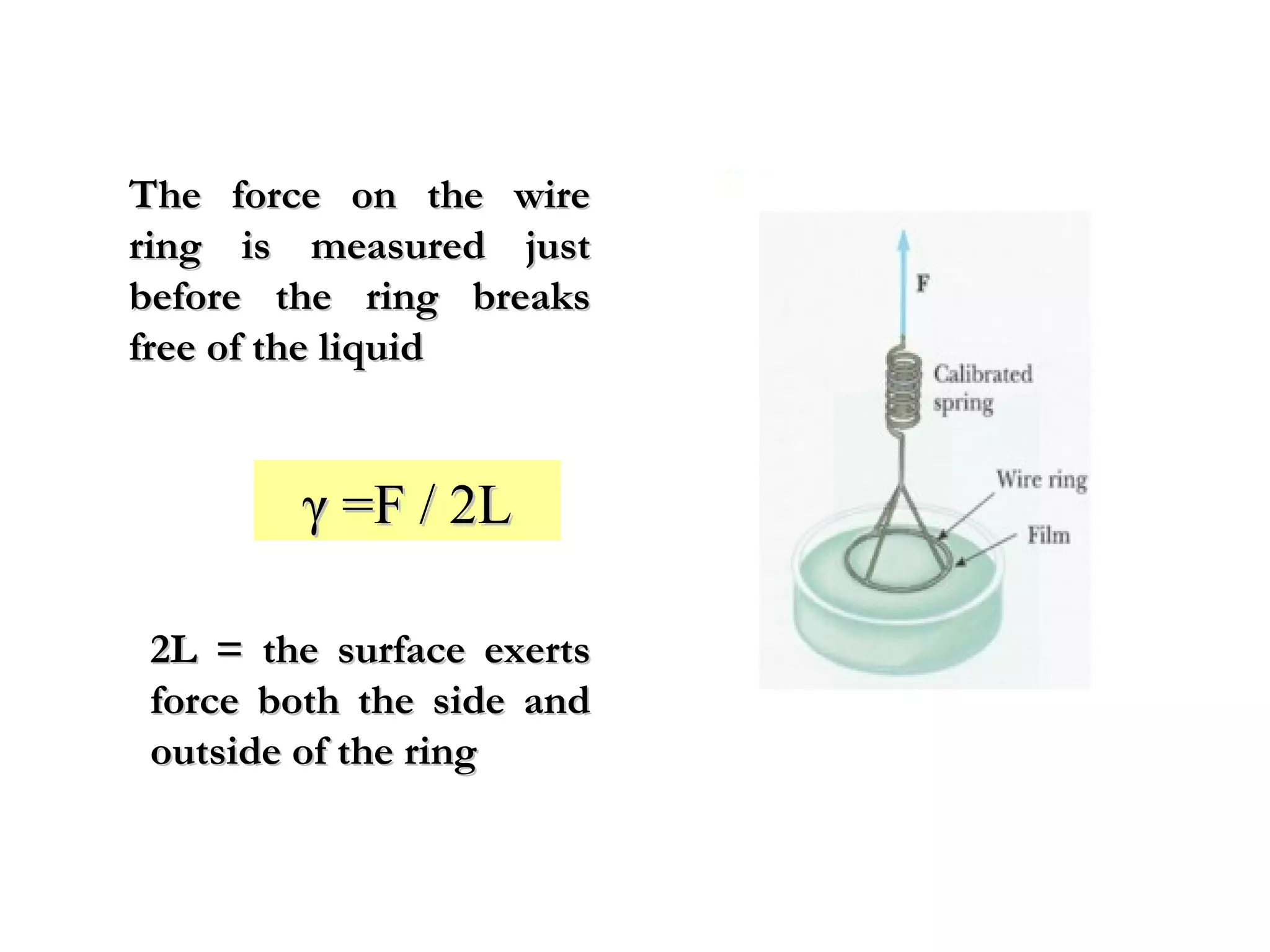
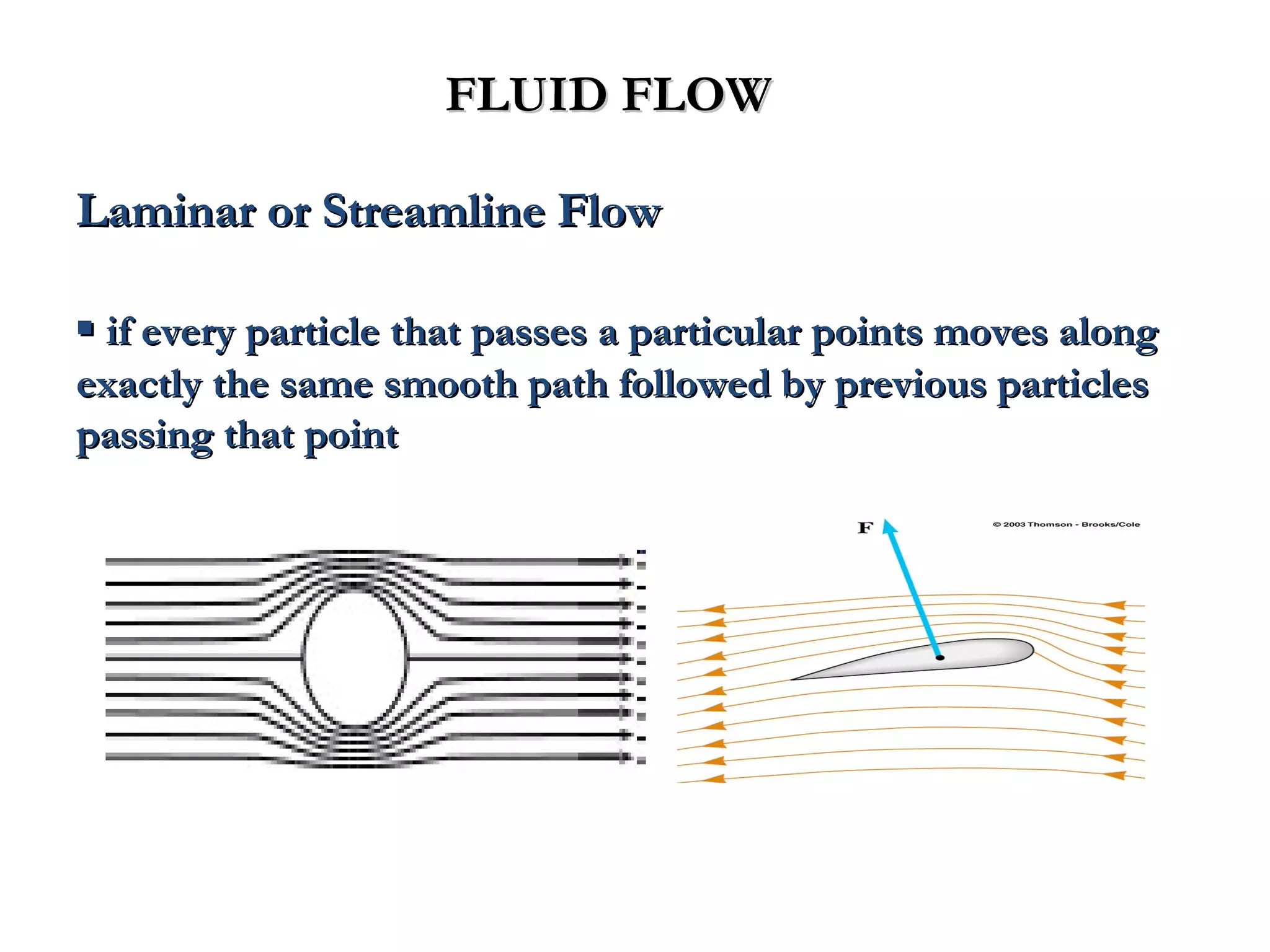
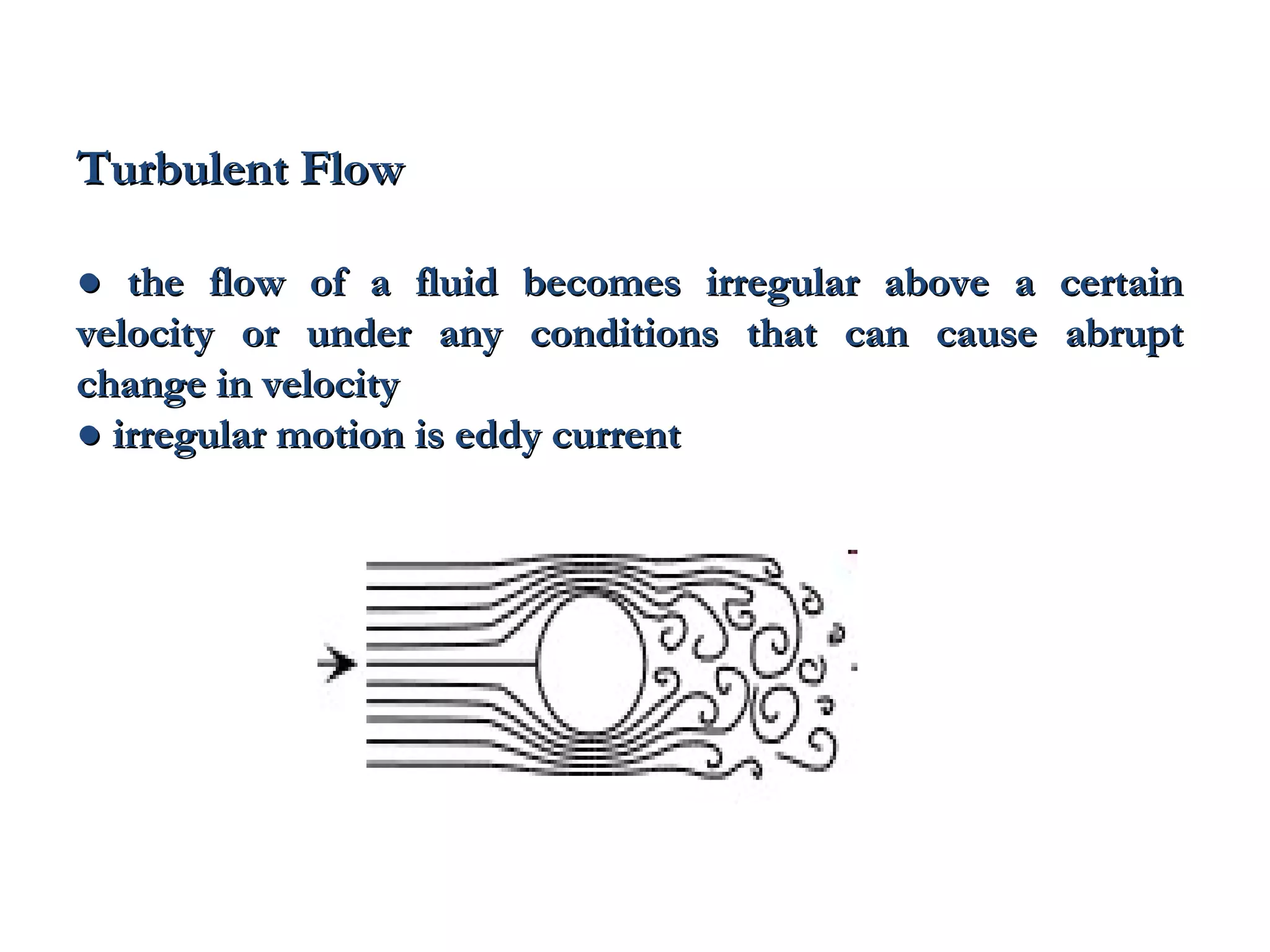
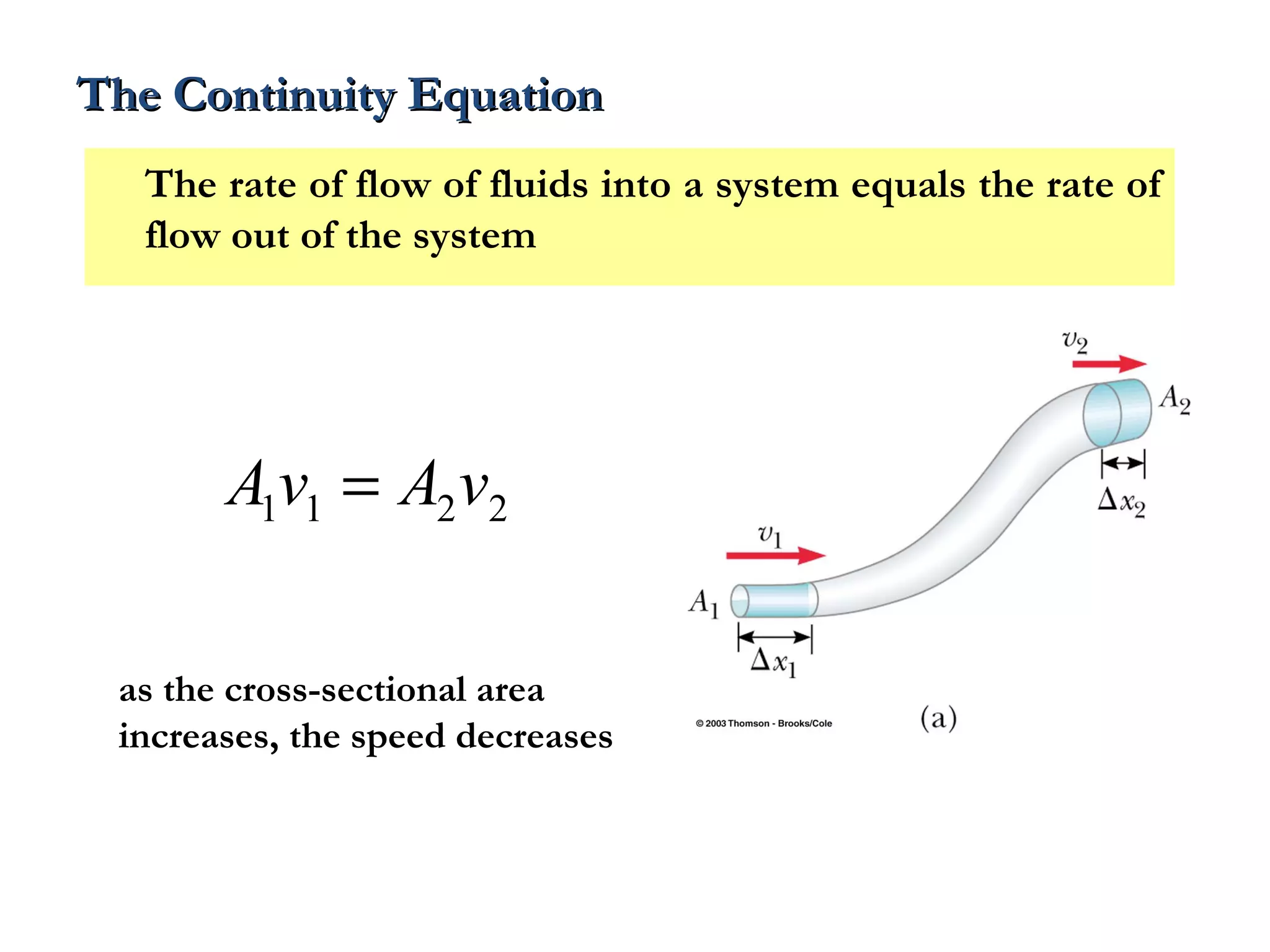
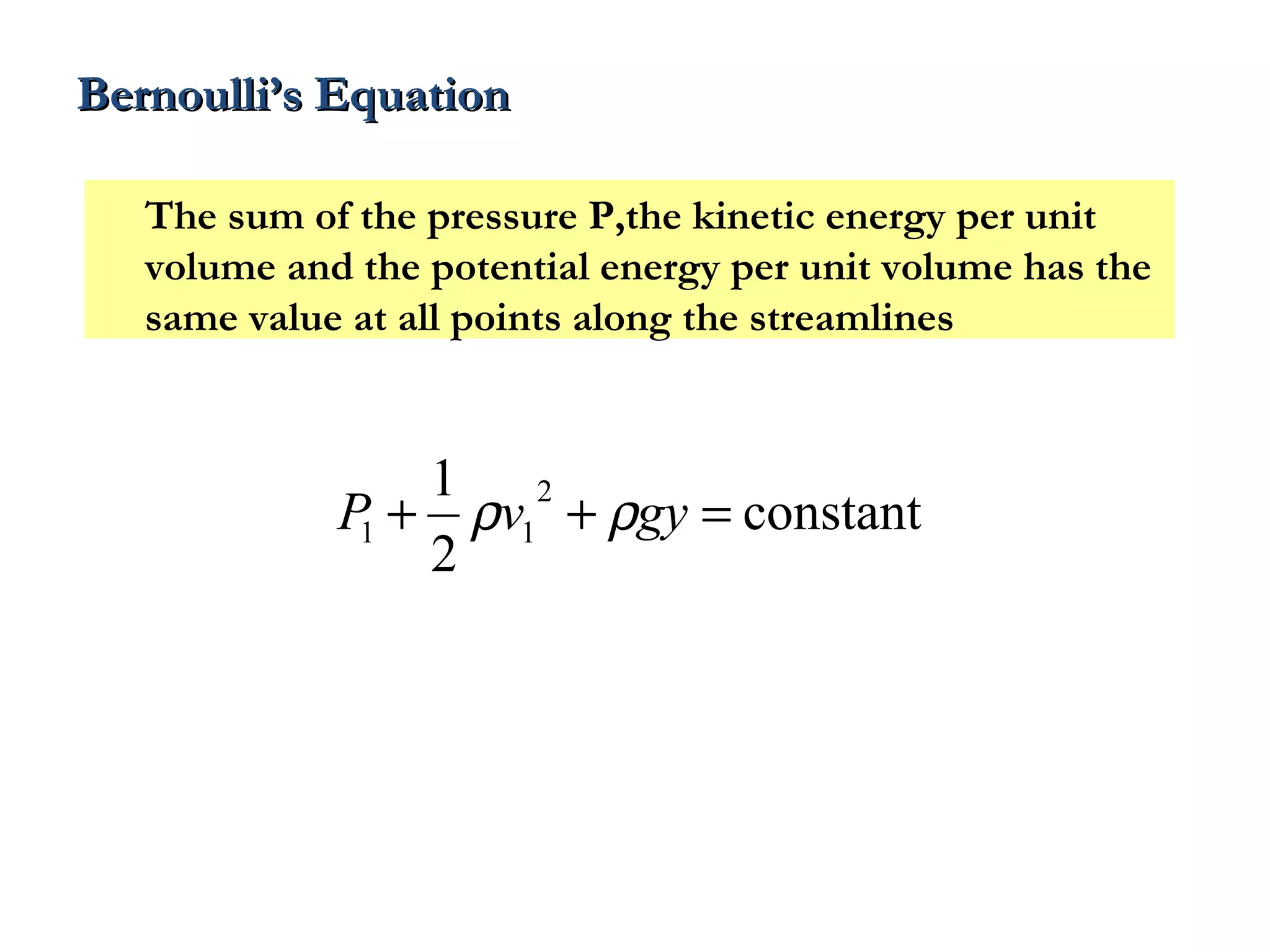
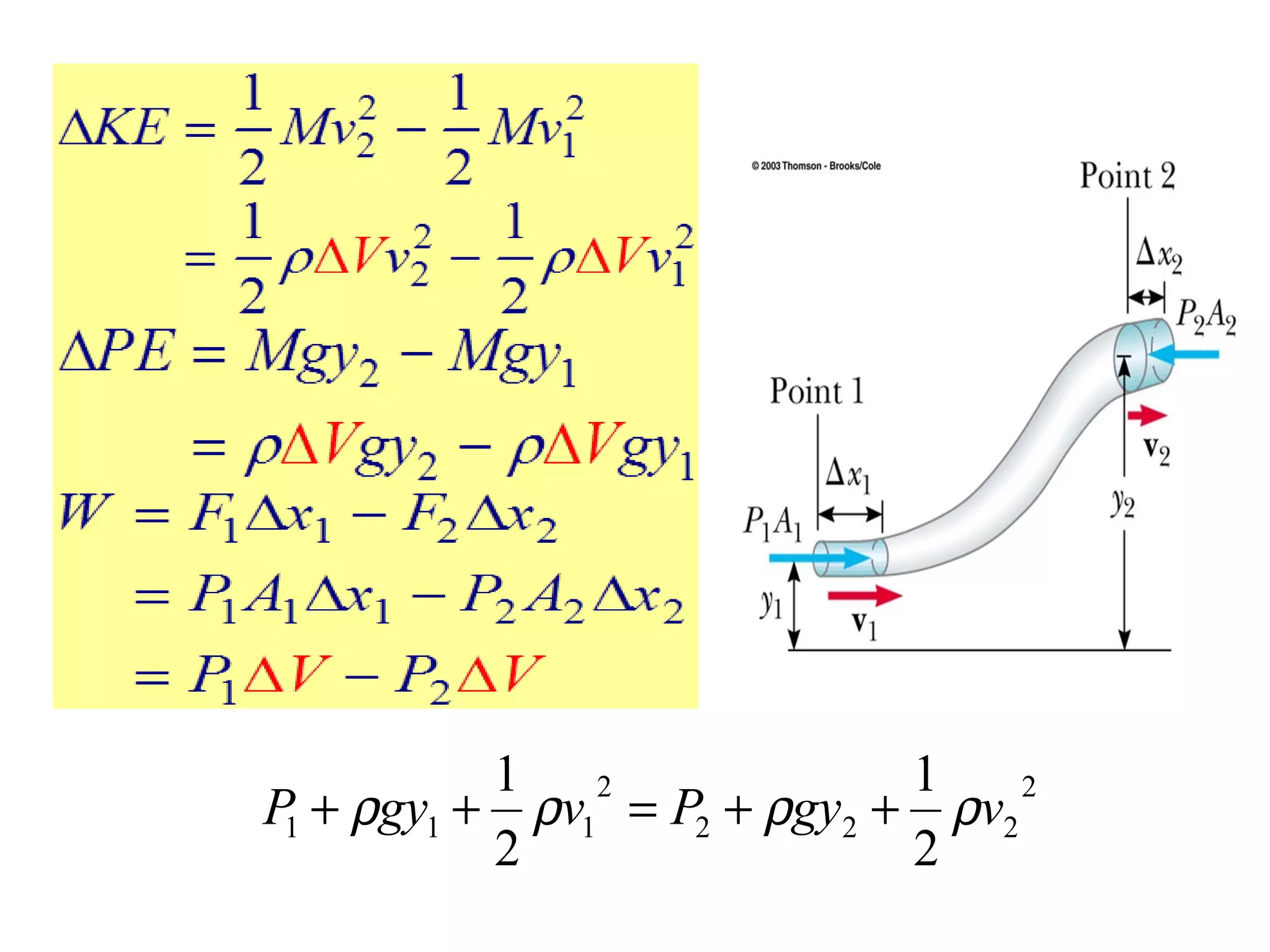
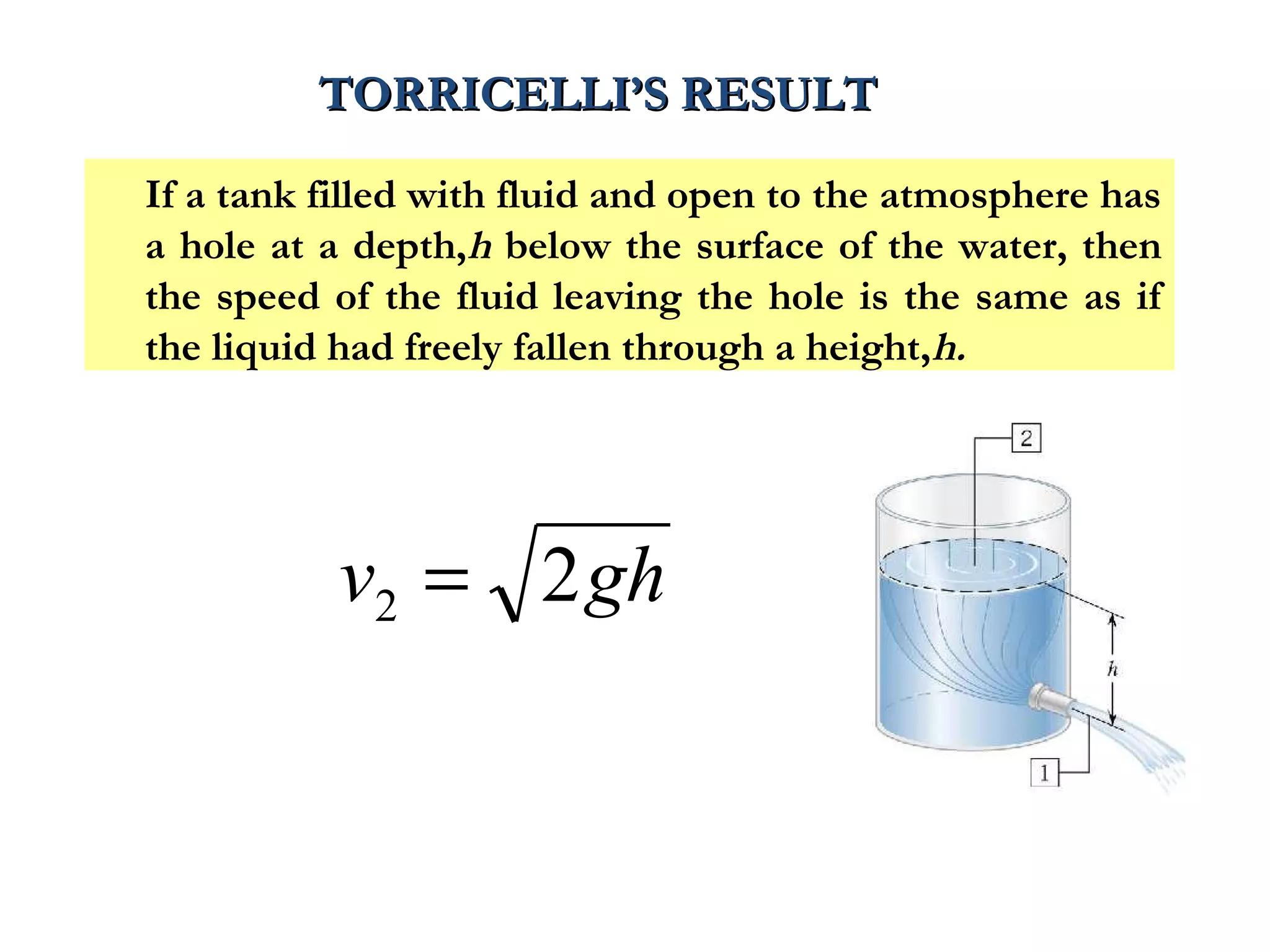
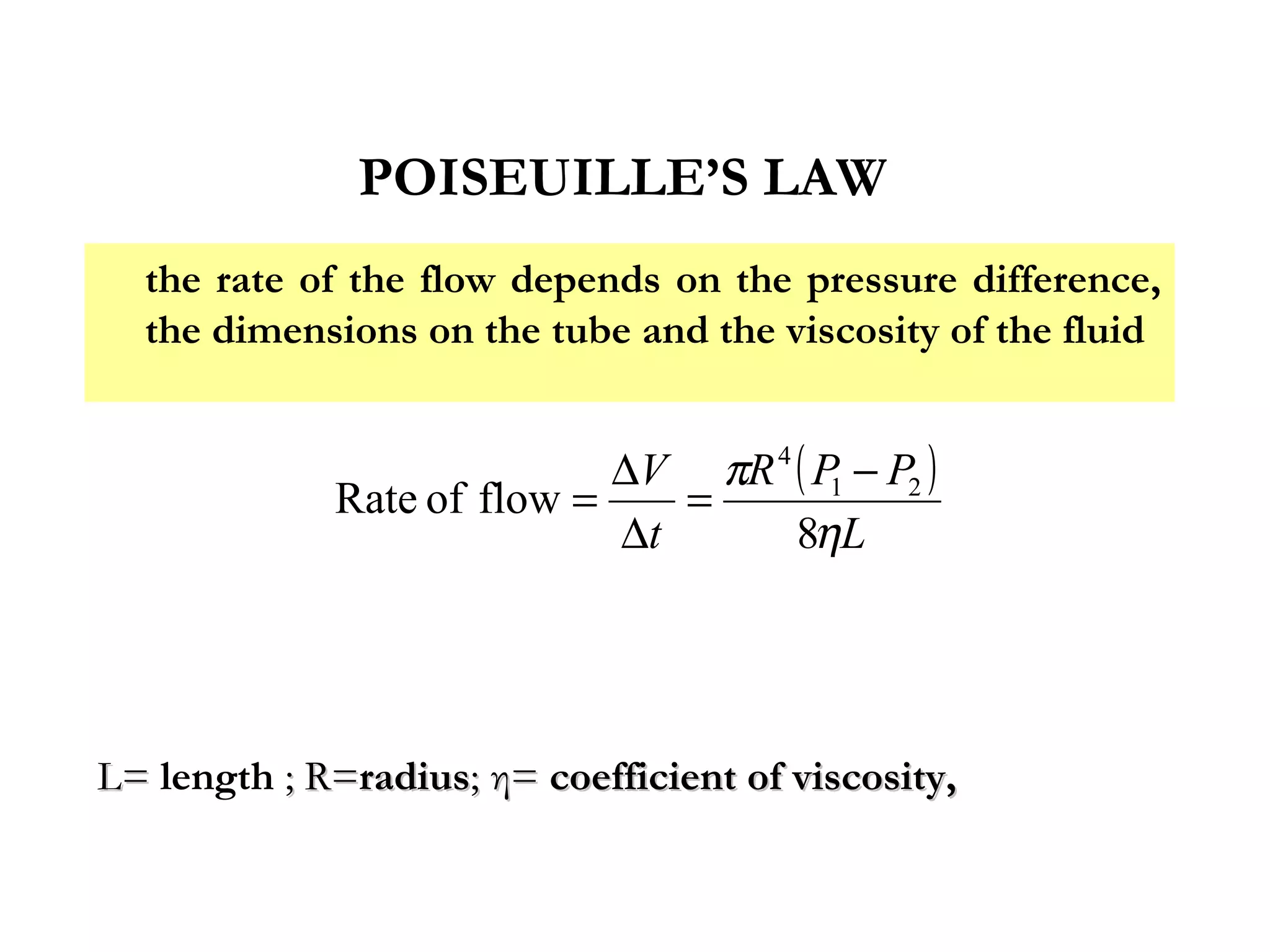
The document discusses the key properties of solids, liquids, and gases. Solids have a definite shape and volume, while liquids have a definite volume but no definite shape and can flow. Gases have no definite shape or volume and expand to fill their container. Solids exhibit long-range order while liquids have weaker binding and shorter-range order. Gases have molecules that are widely separated with disorder prevailing. Intermolecular forces also differ between the three states of matter.