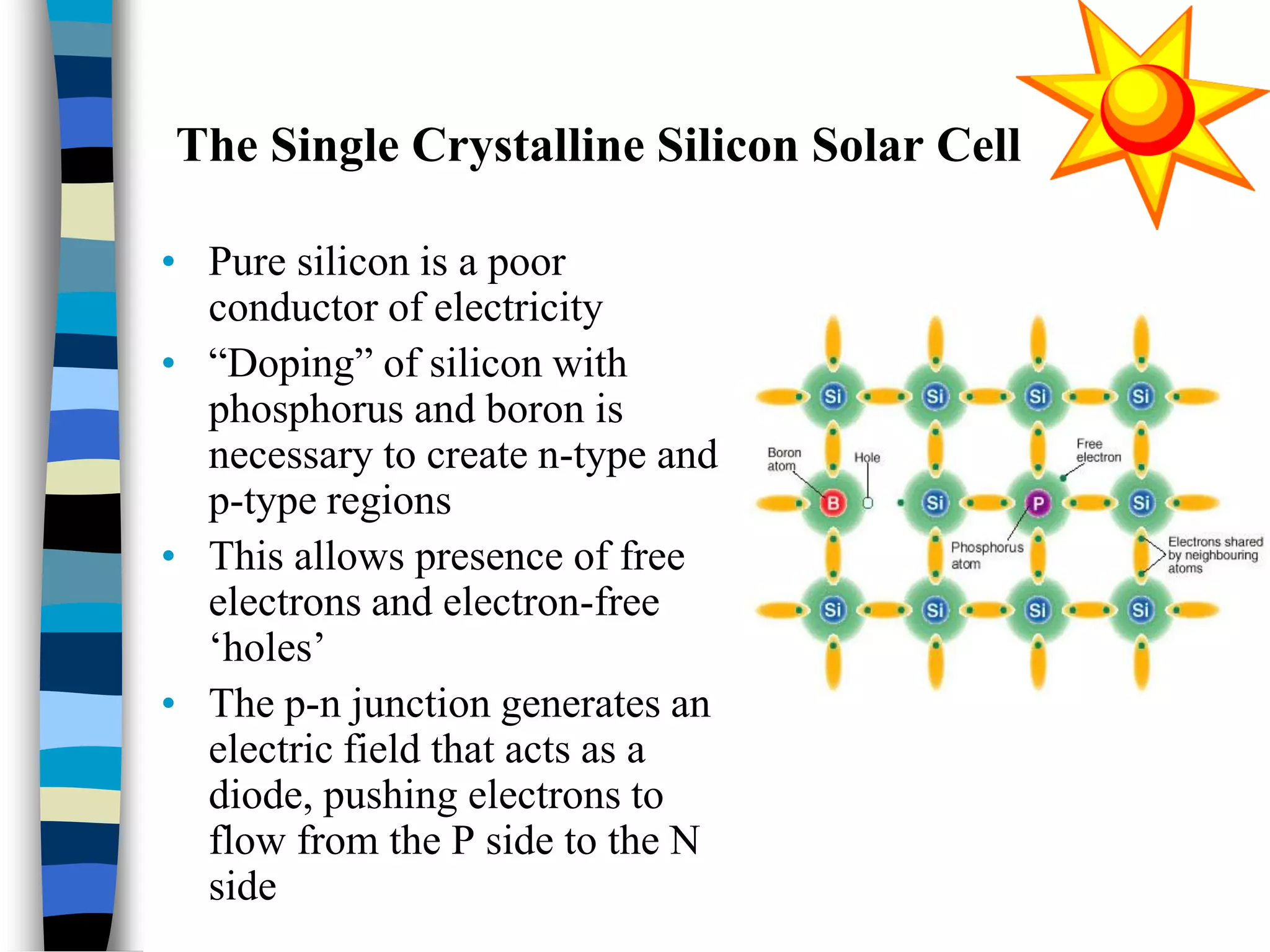
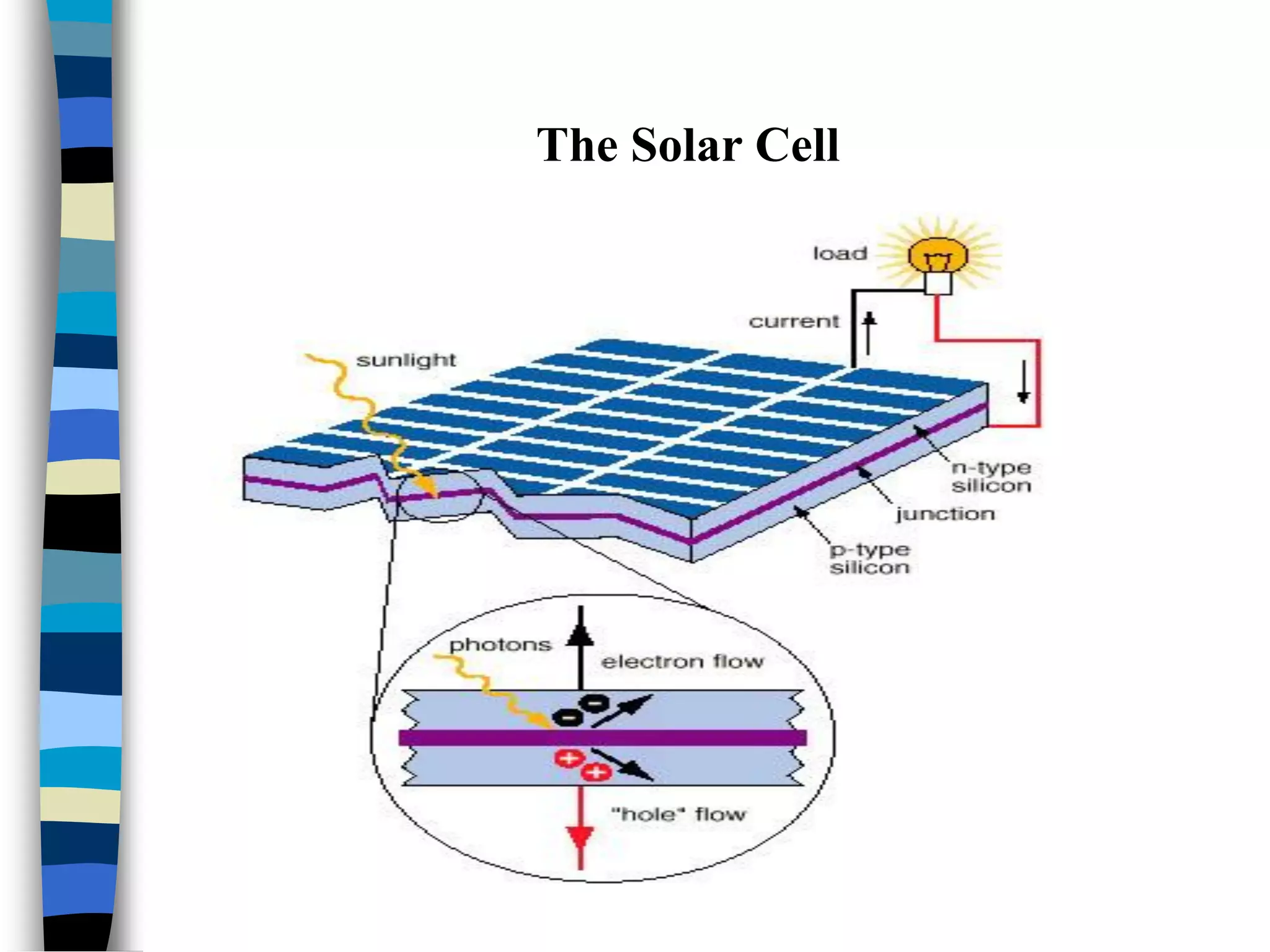
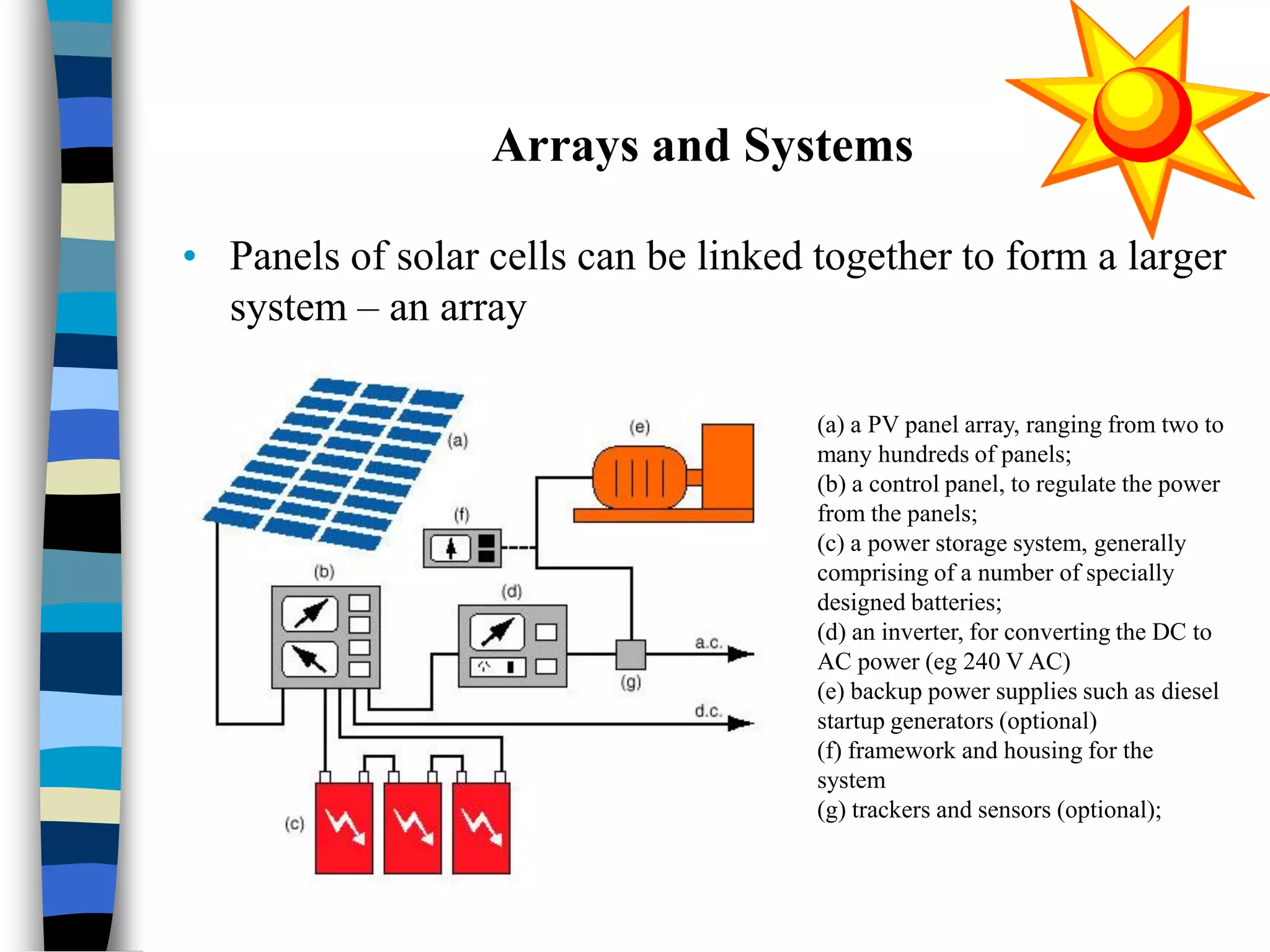
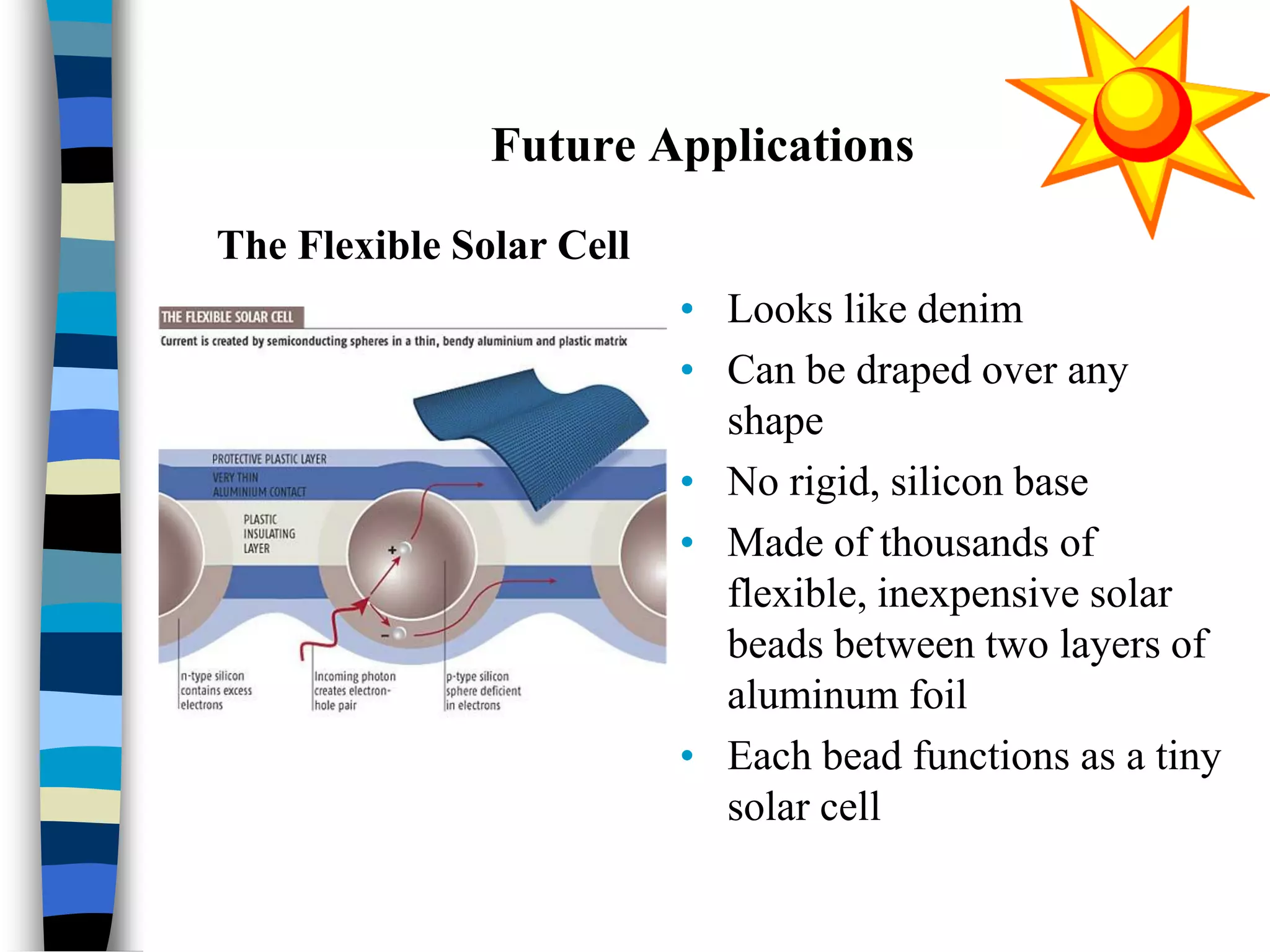
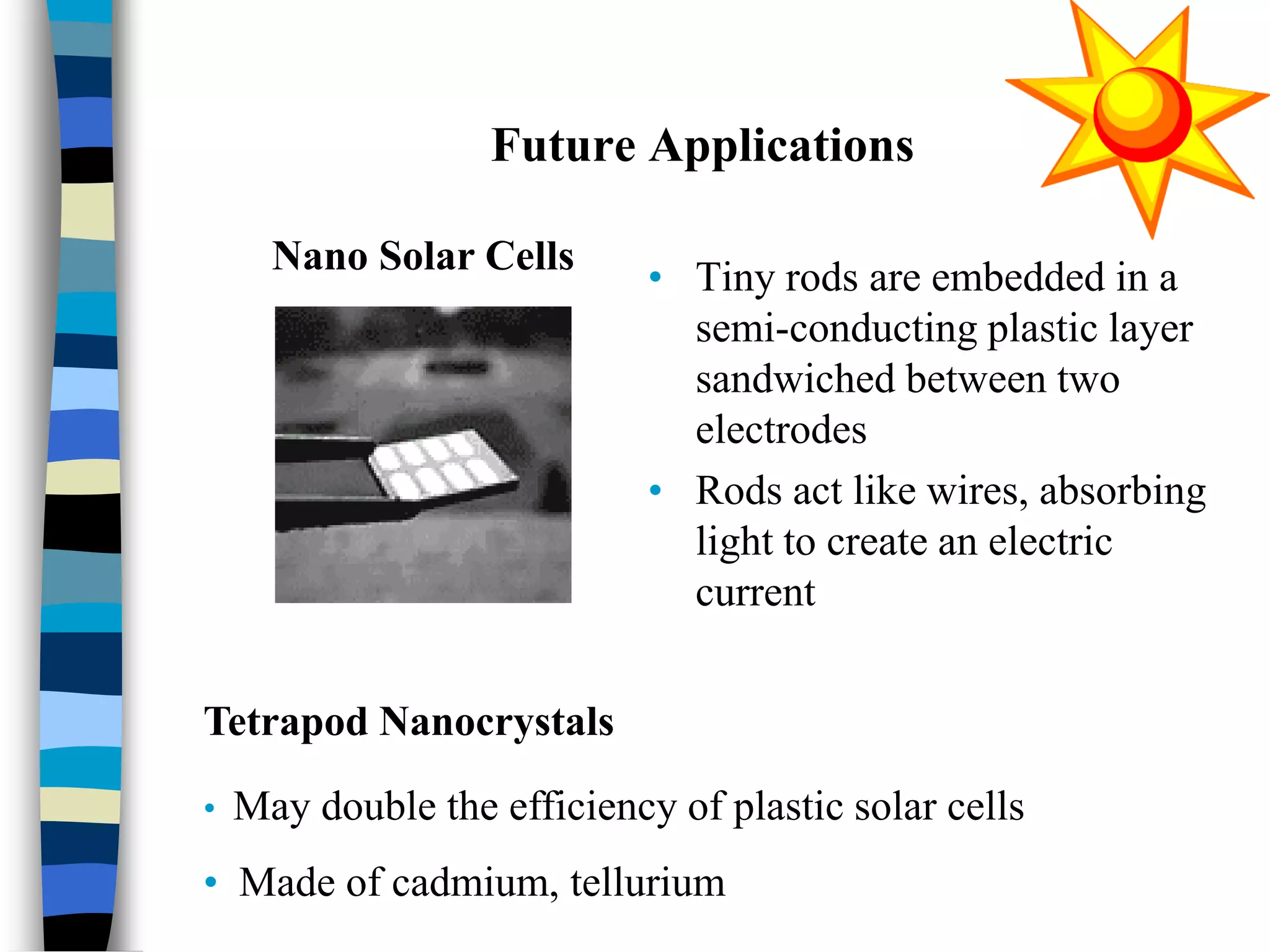
The document discusses solar cells, specifically photovoltaic (PV) cells, which convert sunlight into electricity using semiconductor materials like silicon. It details the functioning, efficiency, and various applications of different types of solar cells, as well as emerging technologies such as flexible and organic solar cells. The summary mentions the cost-effectiveness, sustainability, and low maintenance of solar energy sources, highlighting their increasing market value.