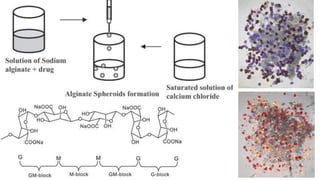
Sodium alginate beads are formed through a process called ionotropic gelation. A sodium alginate solution is dropped into a calcium chloride solution, where the calcium ions cross-link with the alginate chains to form gel beads. The beads are then washed to remove excess calcium ions. Bead properties like size, hardness, and diffusion rate depend on the concentration of sodium alginate used. Sodium alginate beads find applications in drug delivery, cosmetics, biotechnology, and the food industry due to their ability to encapsulate and control the release of various active ingredients.