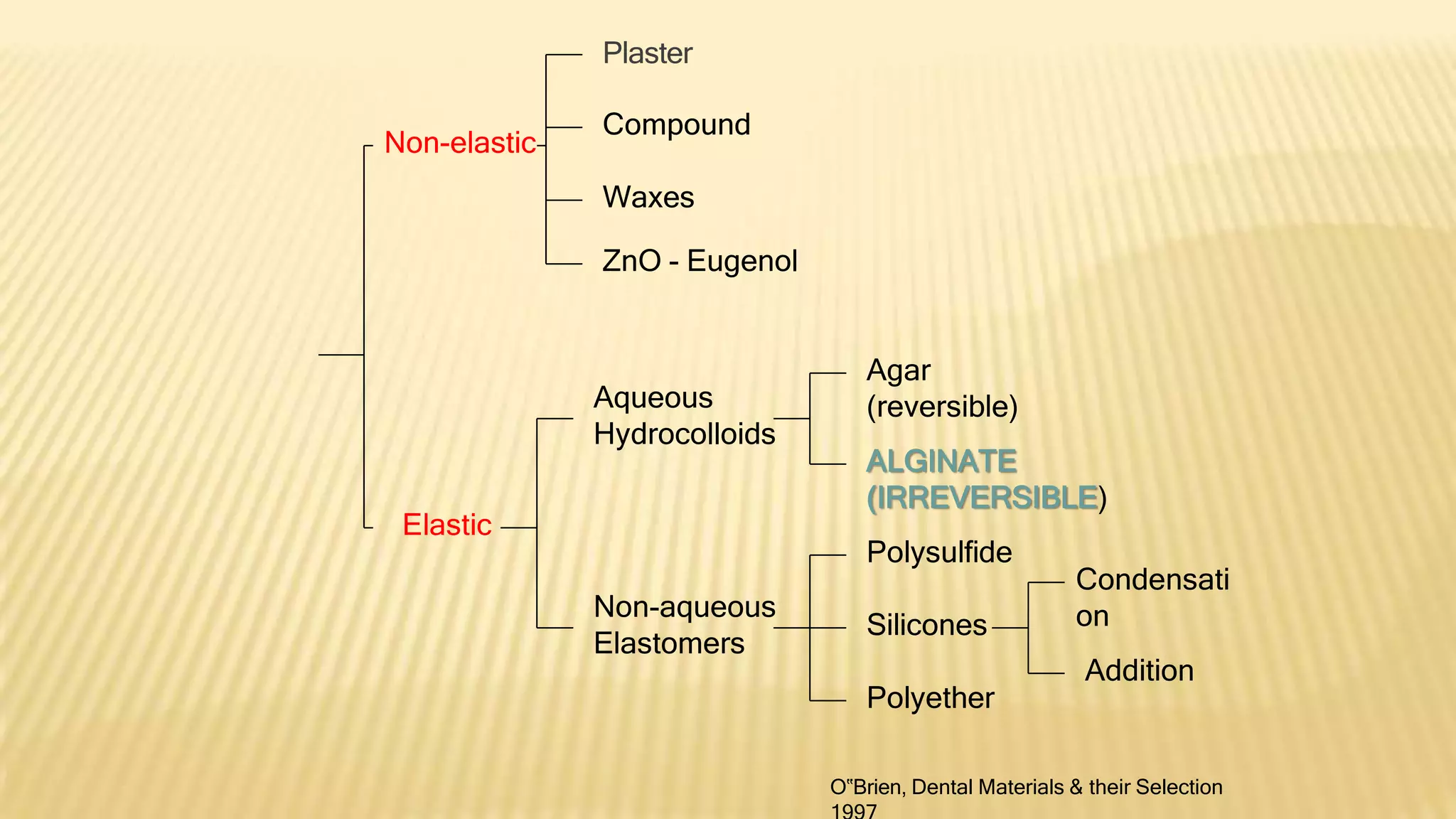
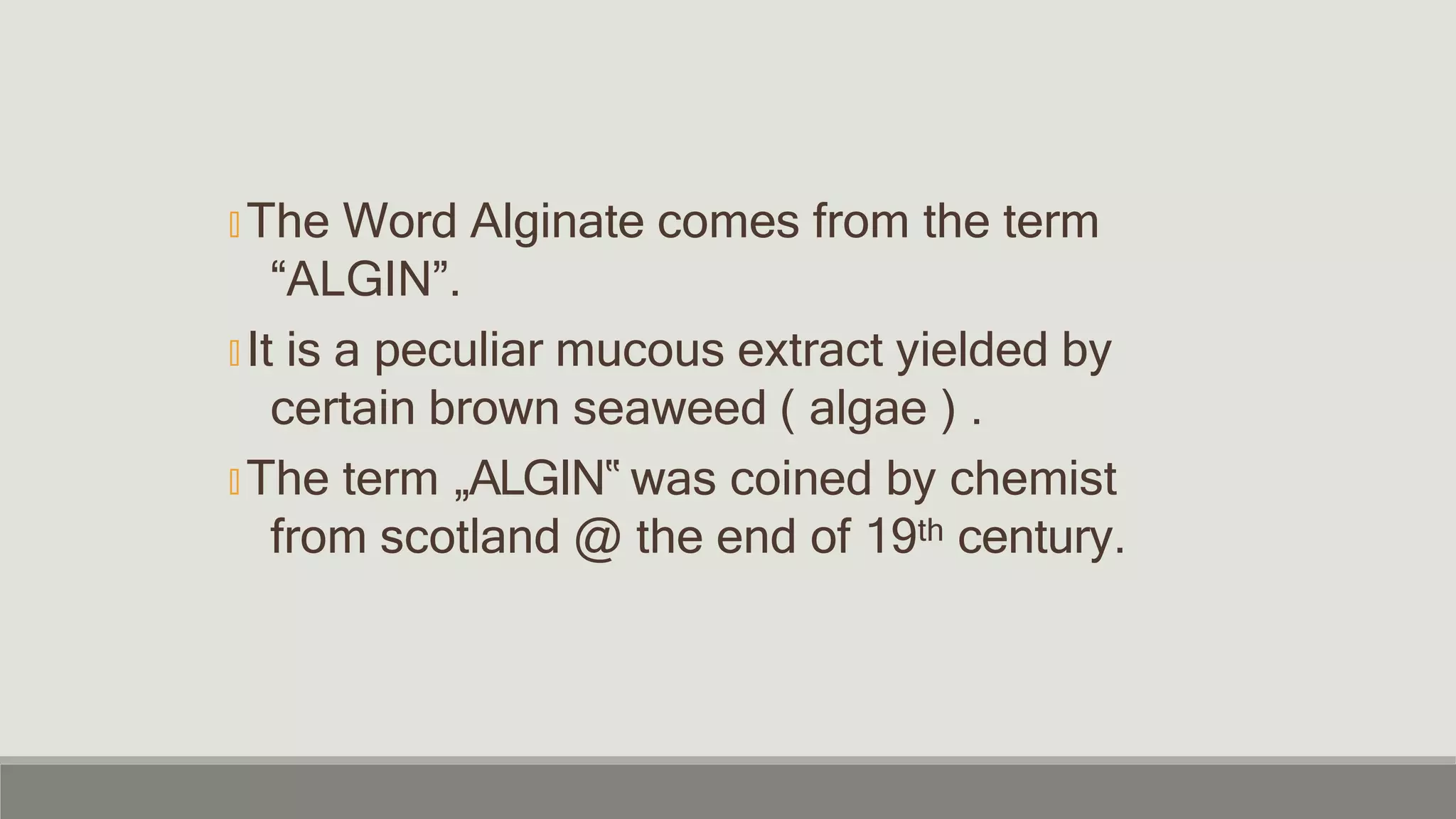
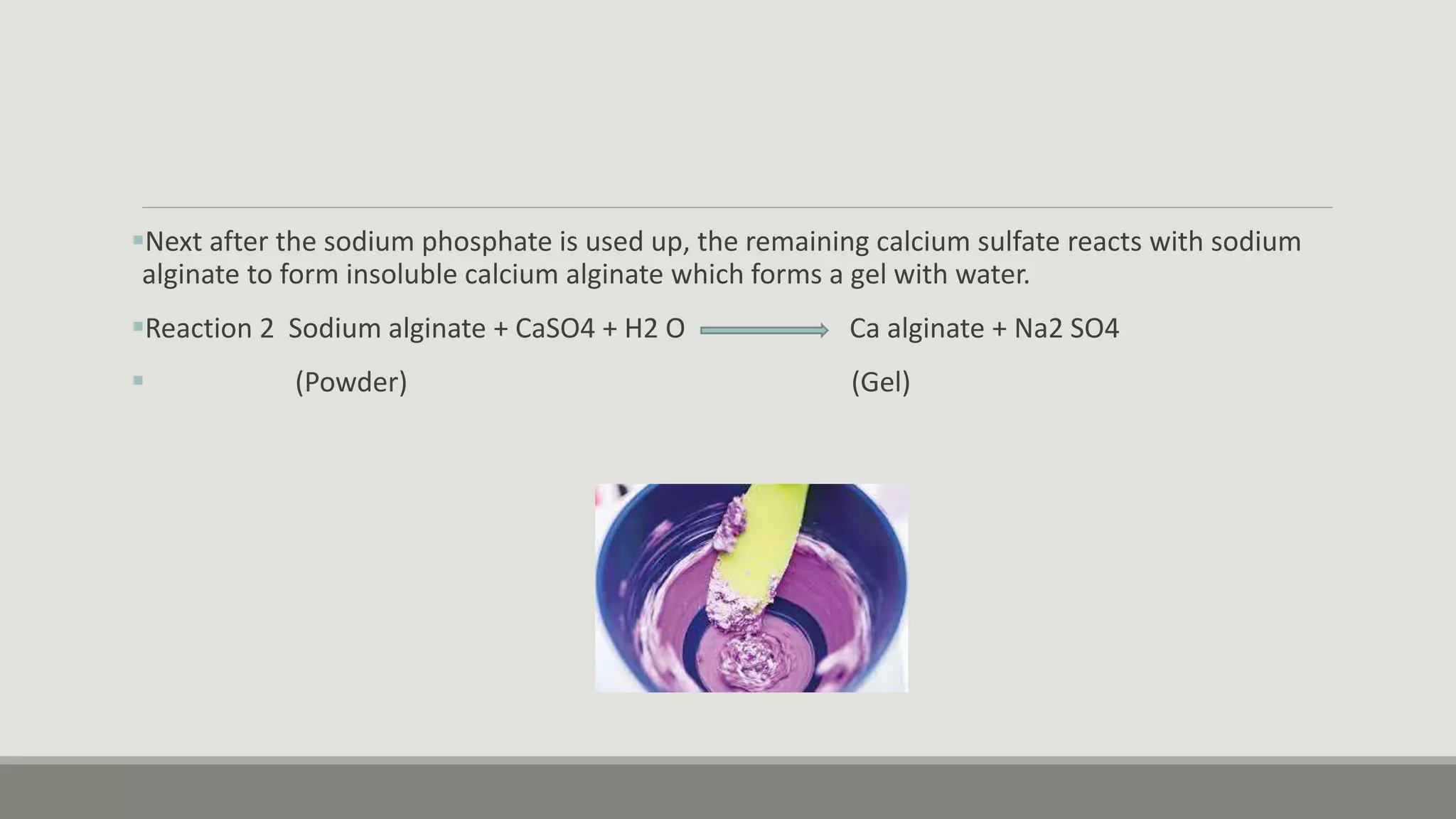
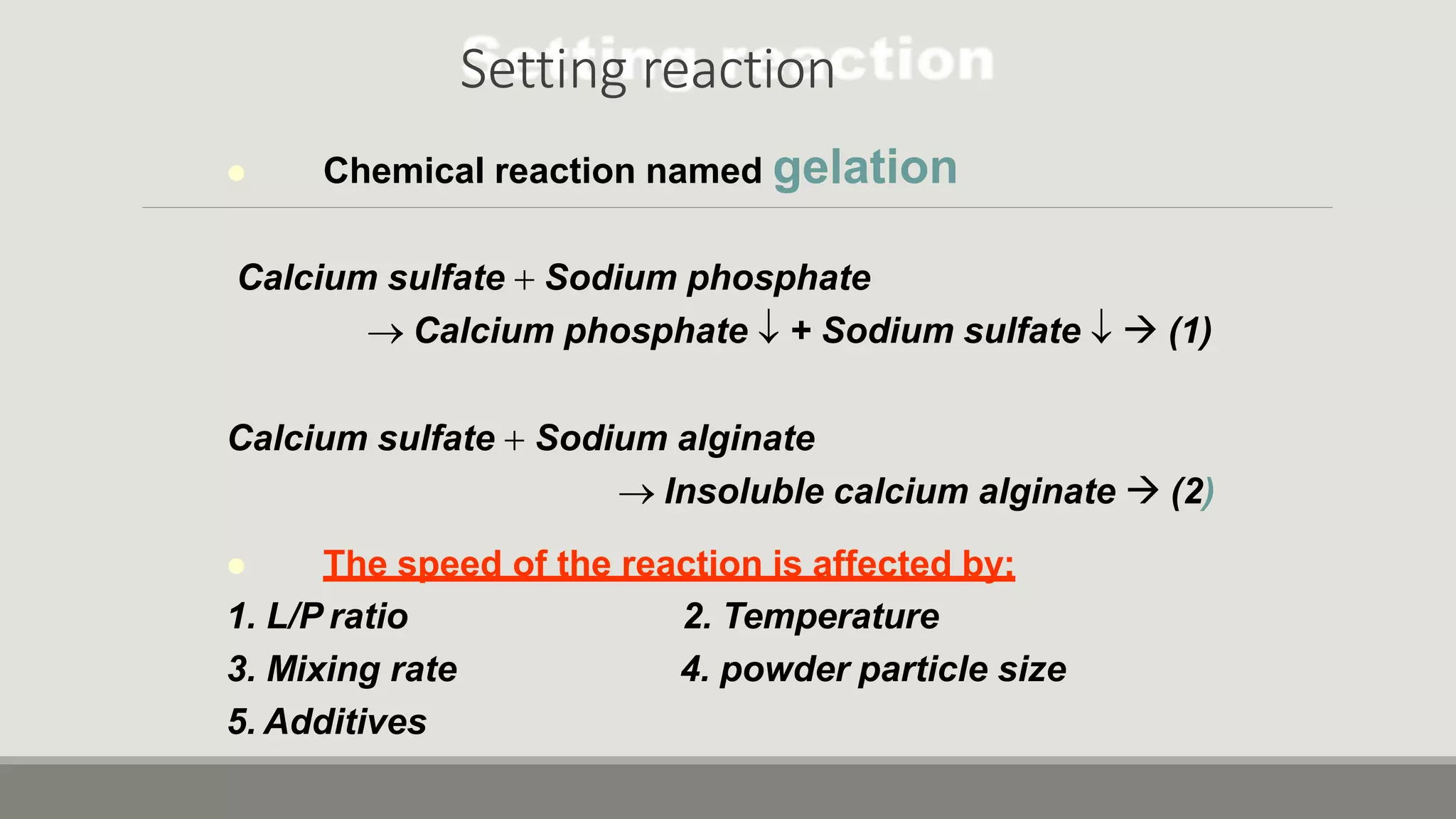
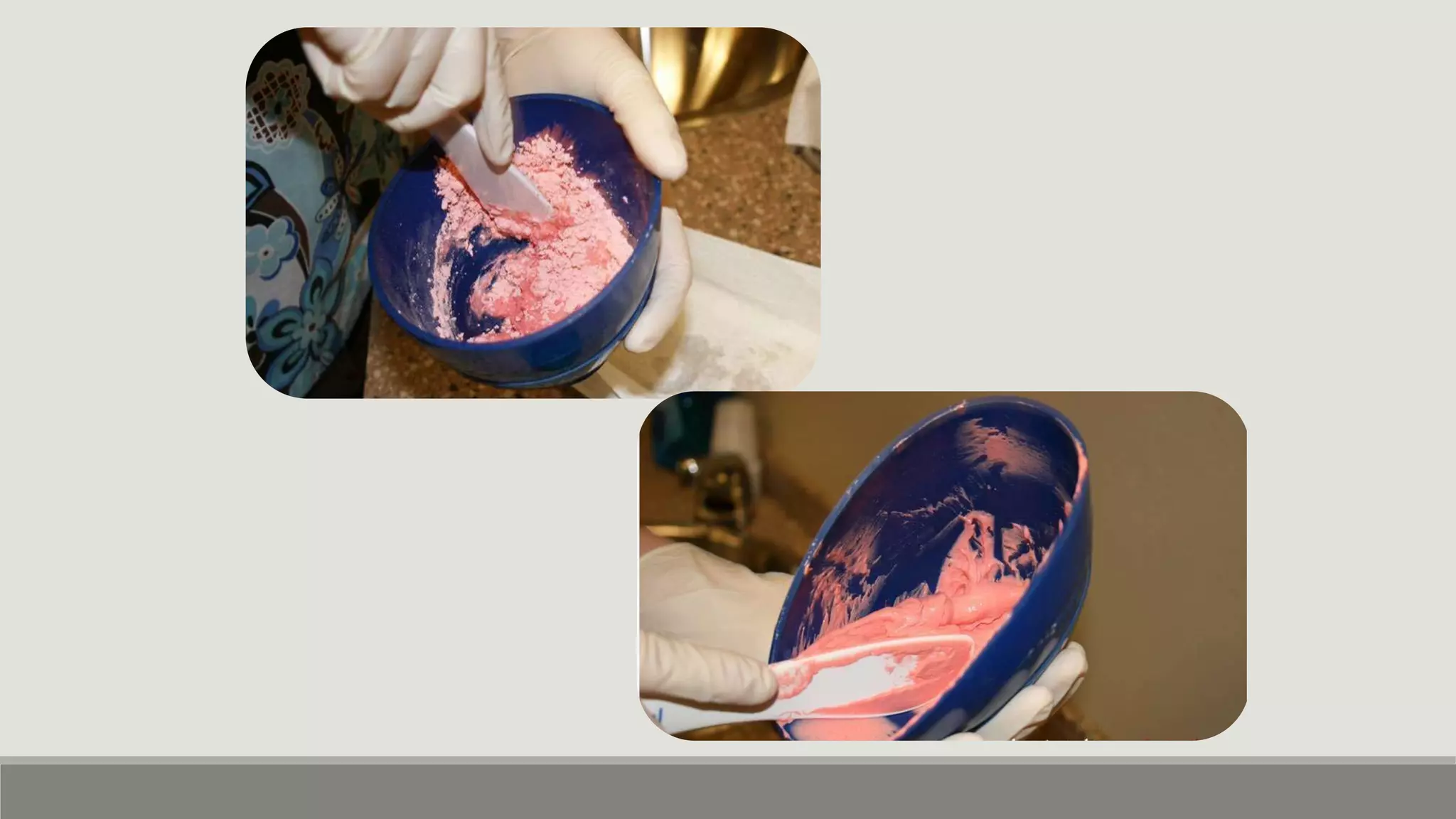
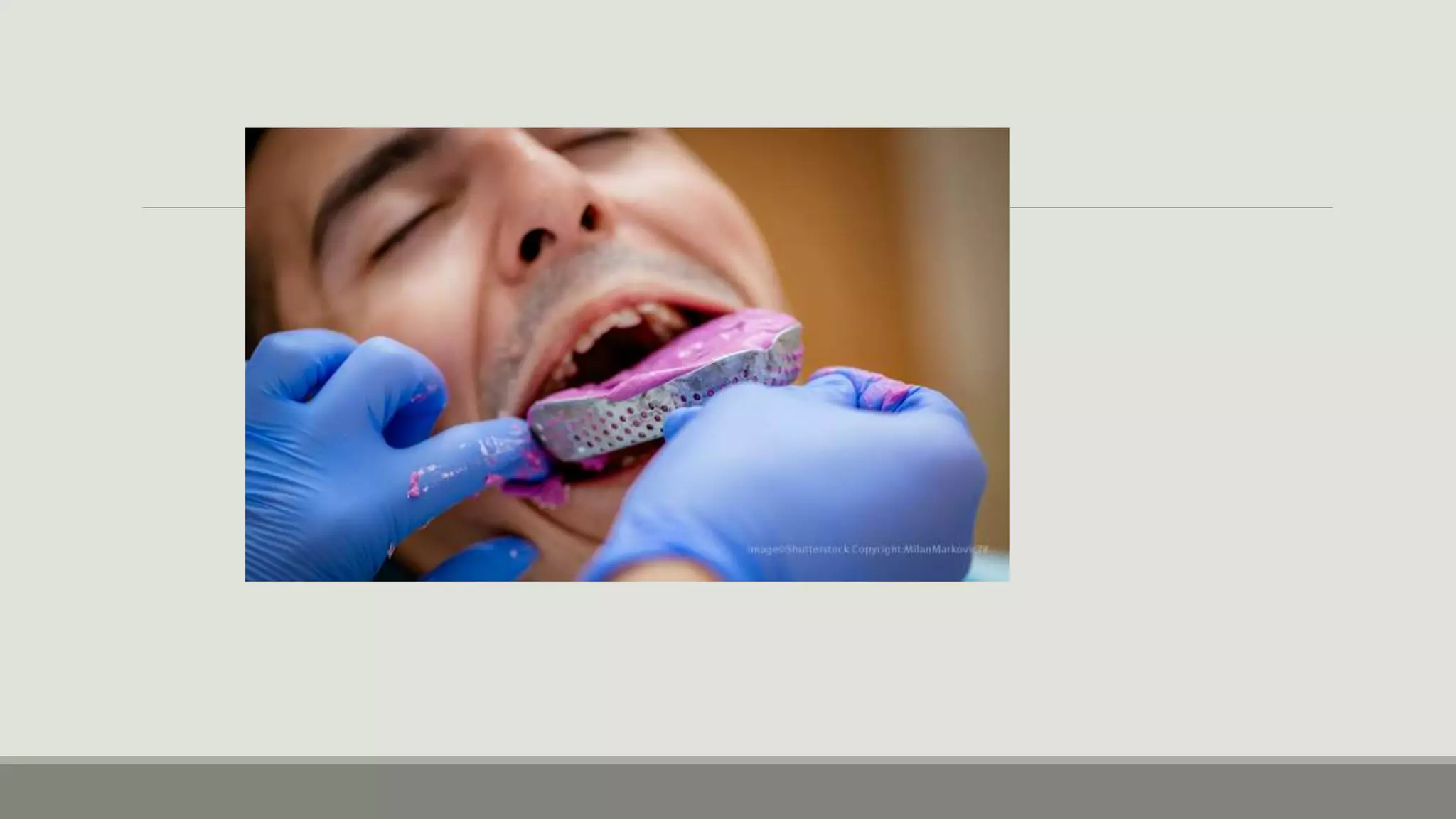
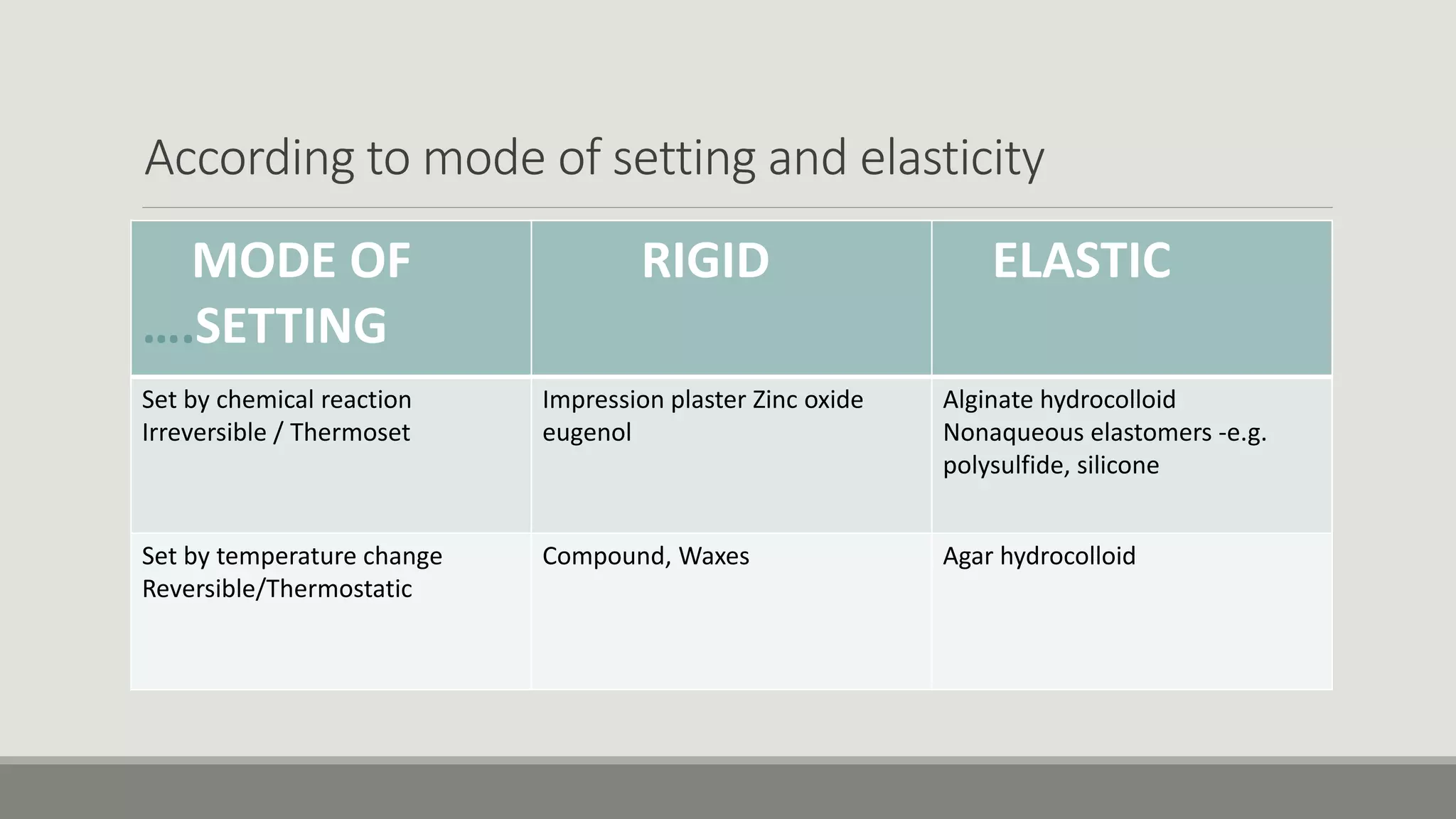
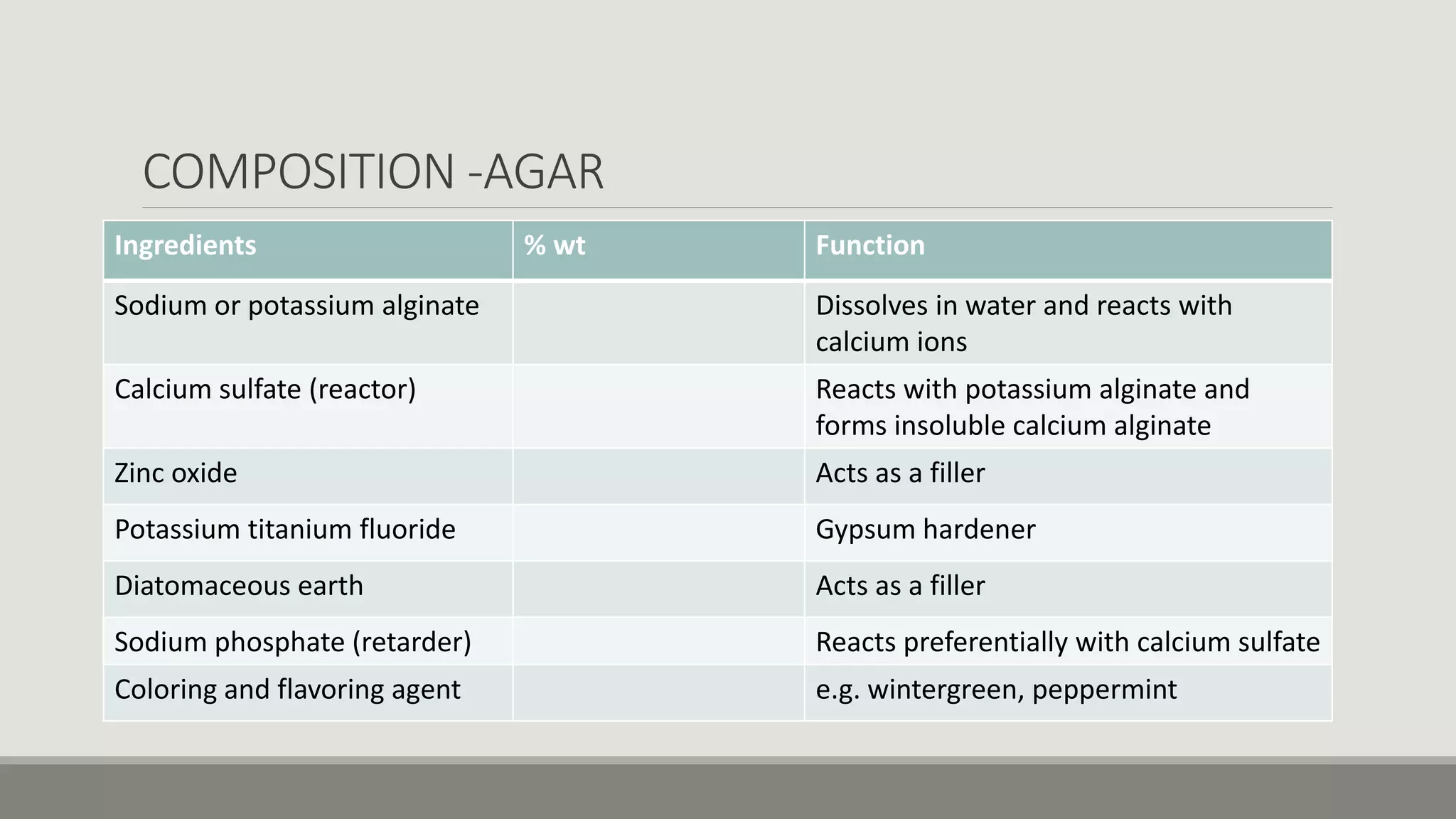
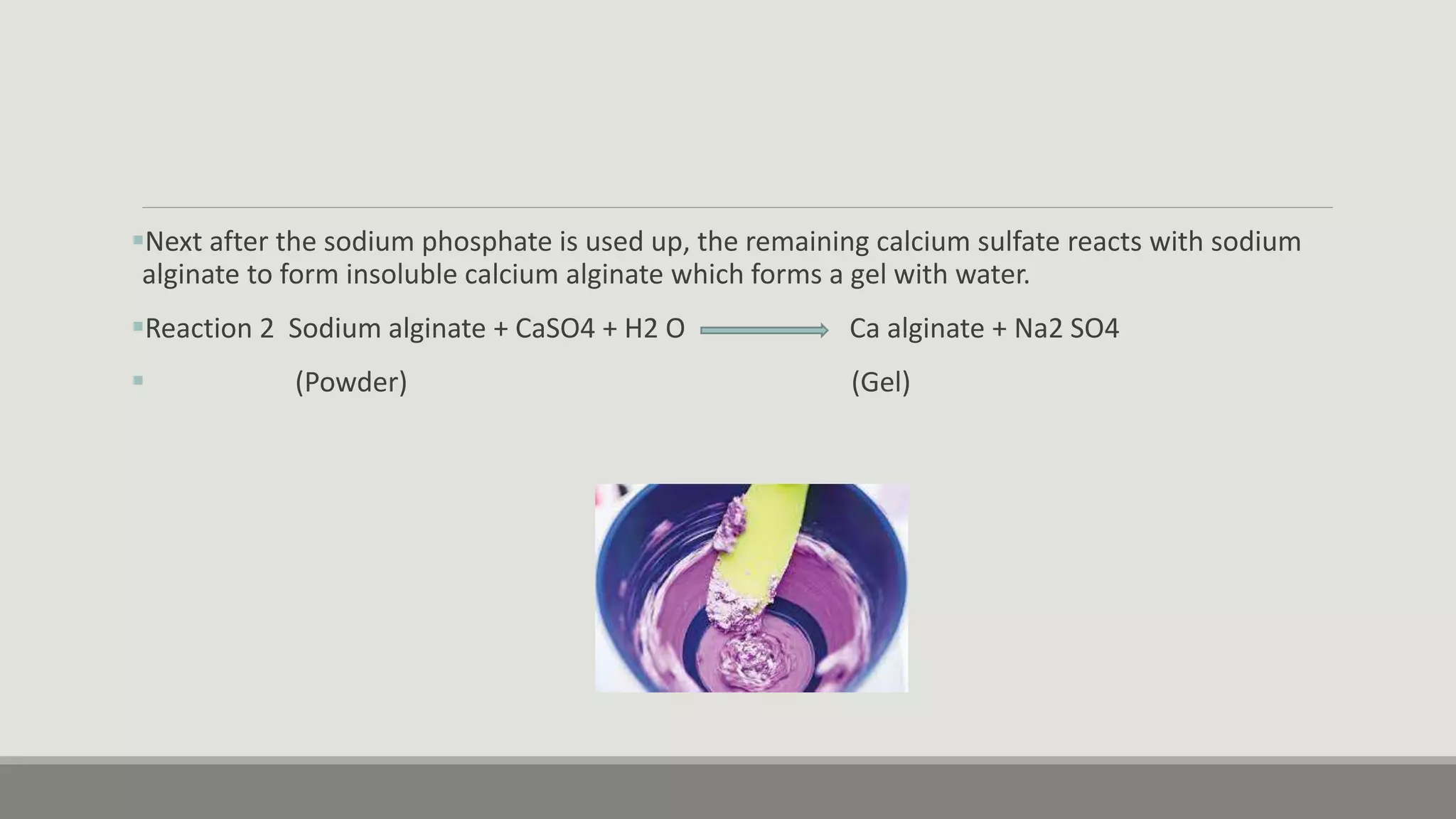
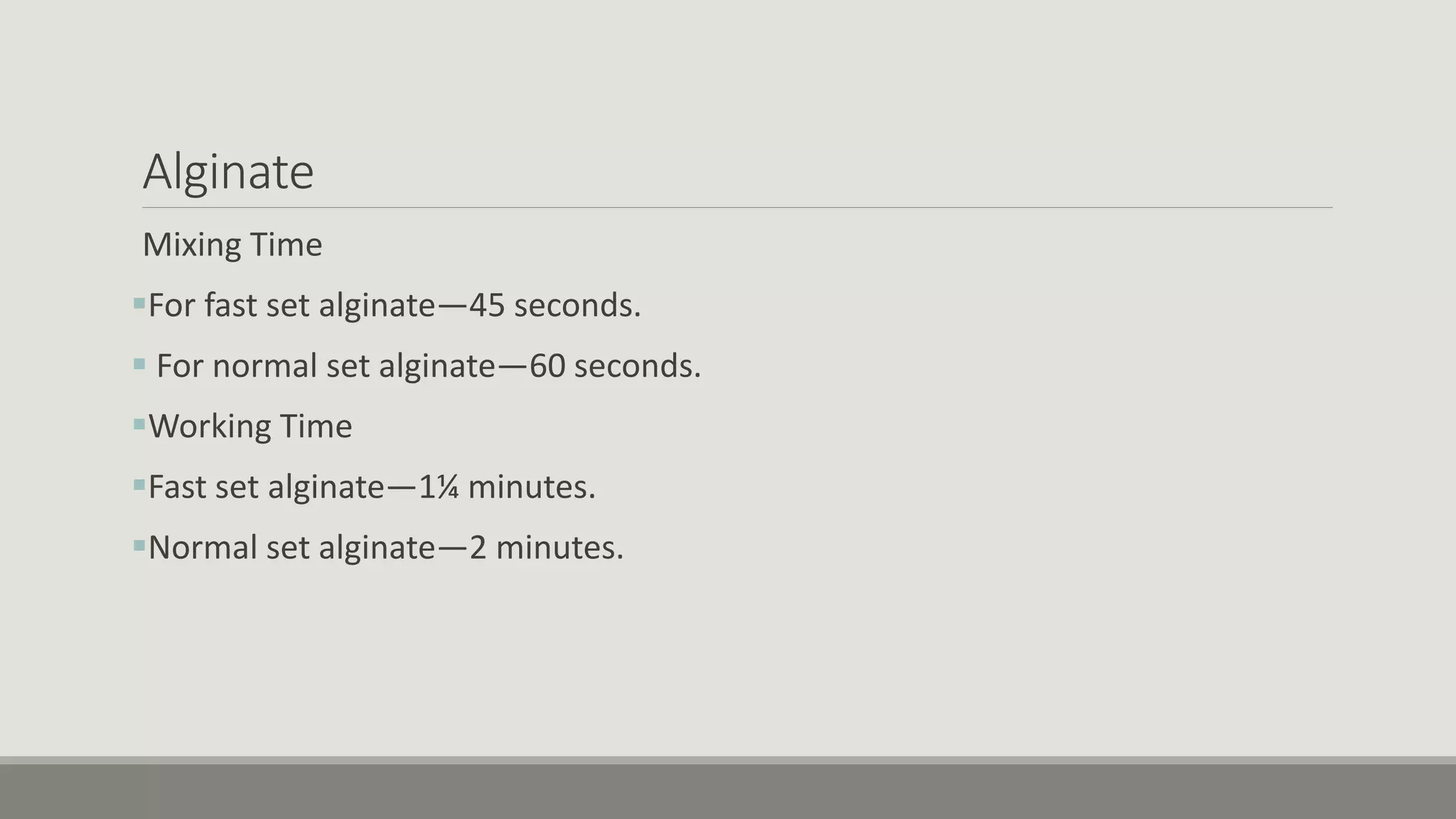
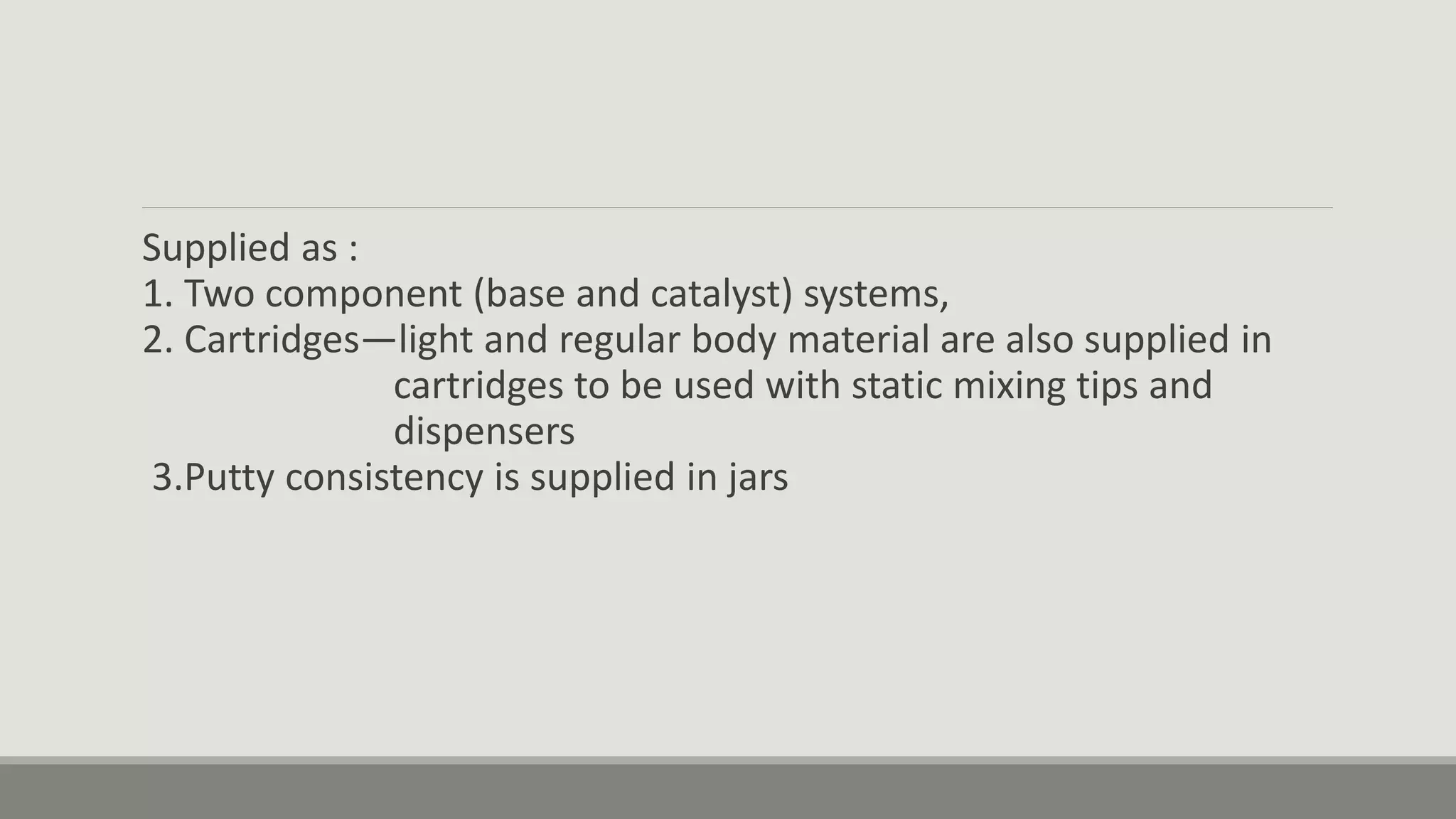
Alginate is an irreversible hydrocolloid impression material made from seaweed. It consists of gelatin particles suspended in water. When the powder and water are mixed, it forms a sol that transitions to a gel through a chemical reaction where soluble alginate reacts with calcium sulfate to form insoluble calcium alginate. This reaction is delayed by adding trisodium phosphate. Alginate has adequate working and setting times and is used for impressions where there are undercuts or excess saliva. It provides detailed impressions but is prone to dimensional changes with moisture changes.