The document discusses the key concepts related to the rate of a chemical reaction:
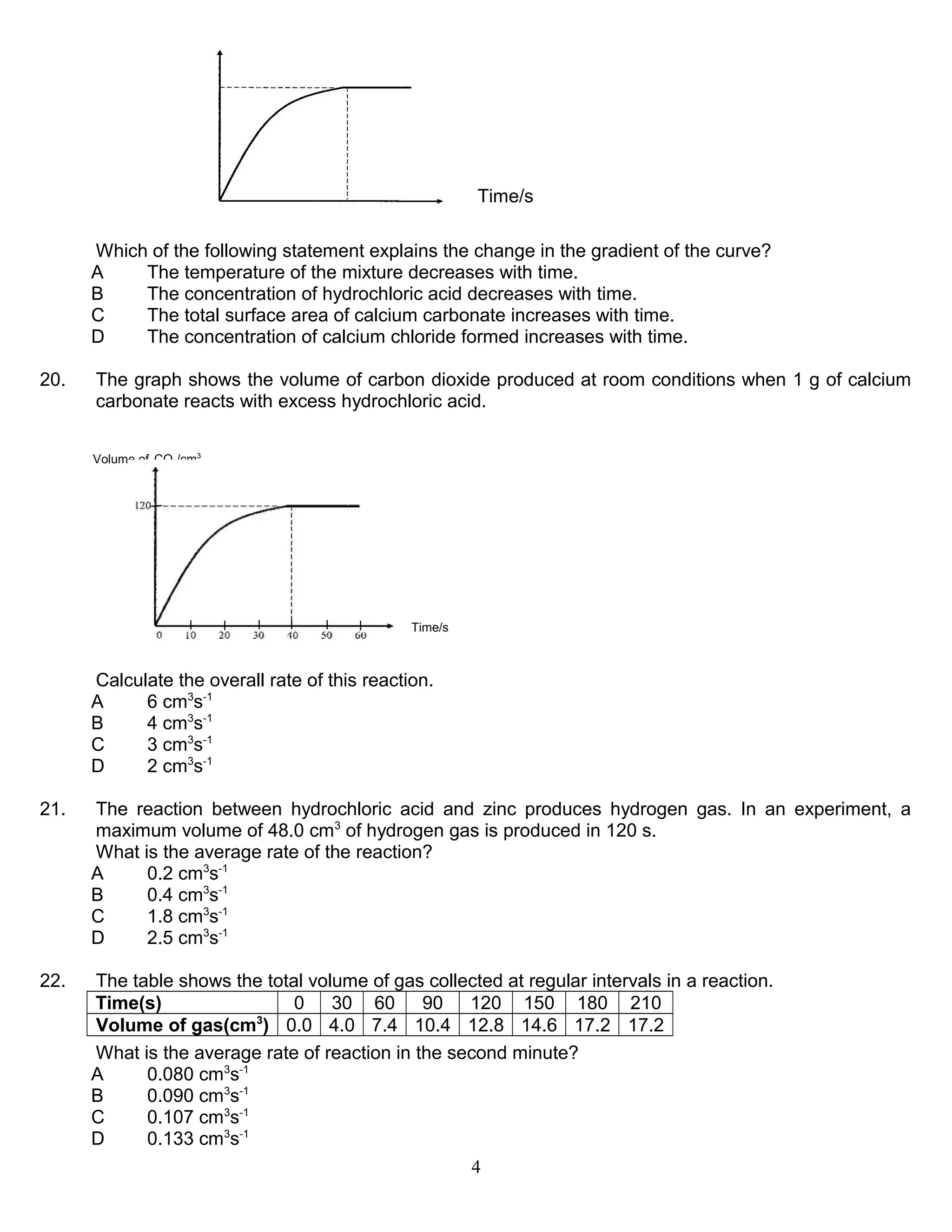
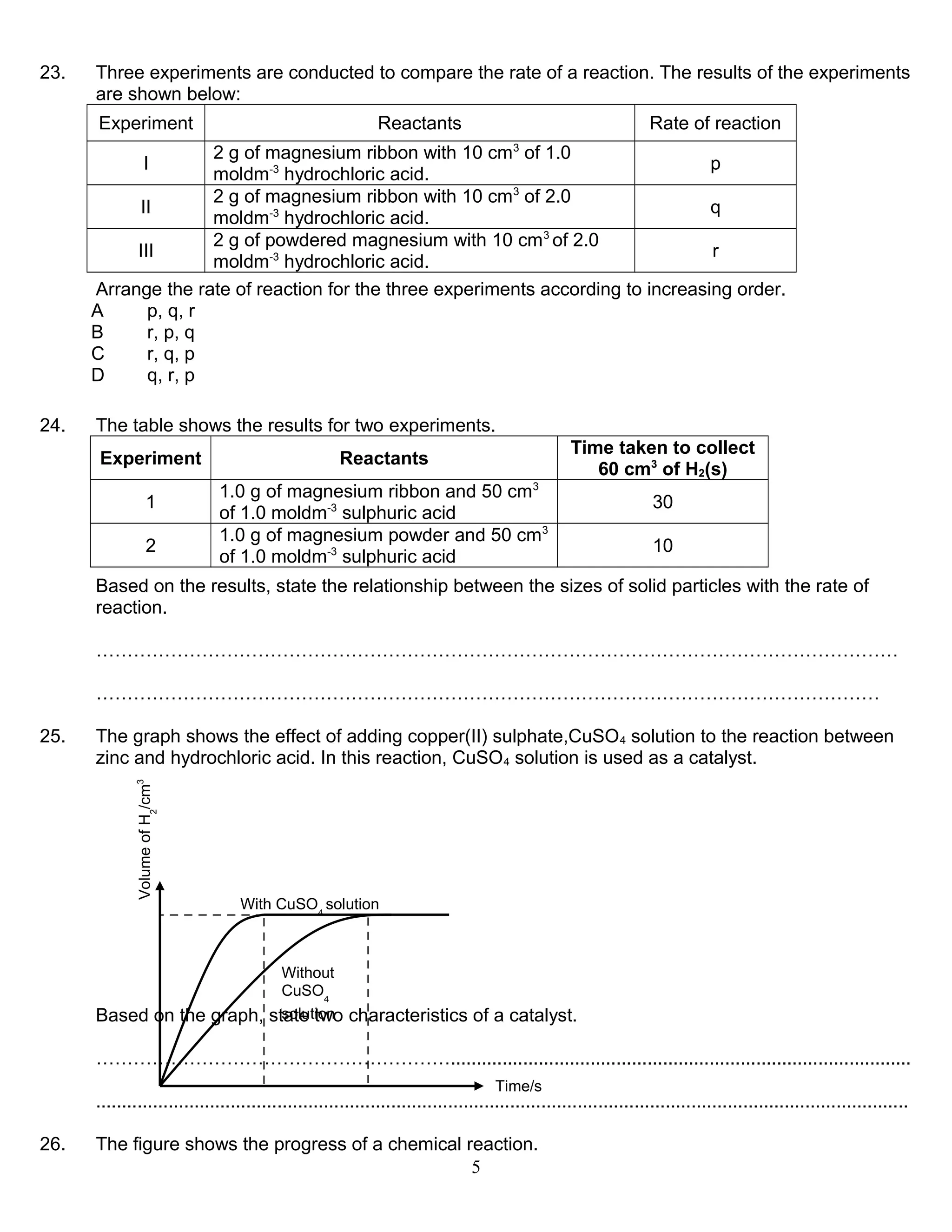
1. The rate of reaction is affected by factors like concentration, temperature, surface area, and presence of a catalyst. A catalyst increases the rate by lowering the activation energy without being consumed in the reaction.
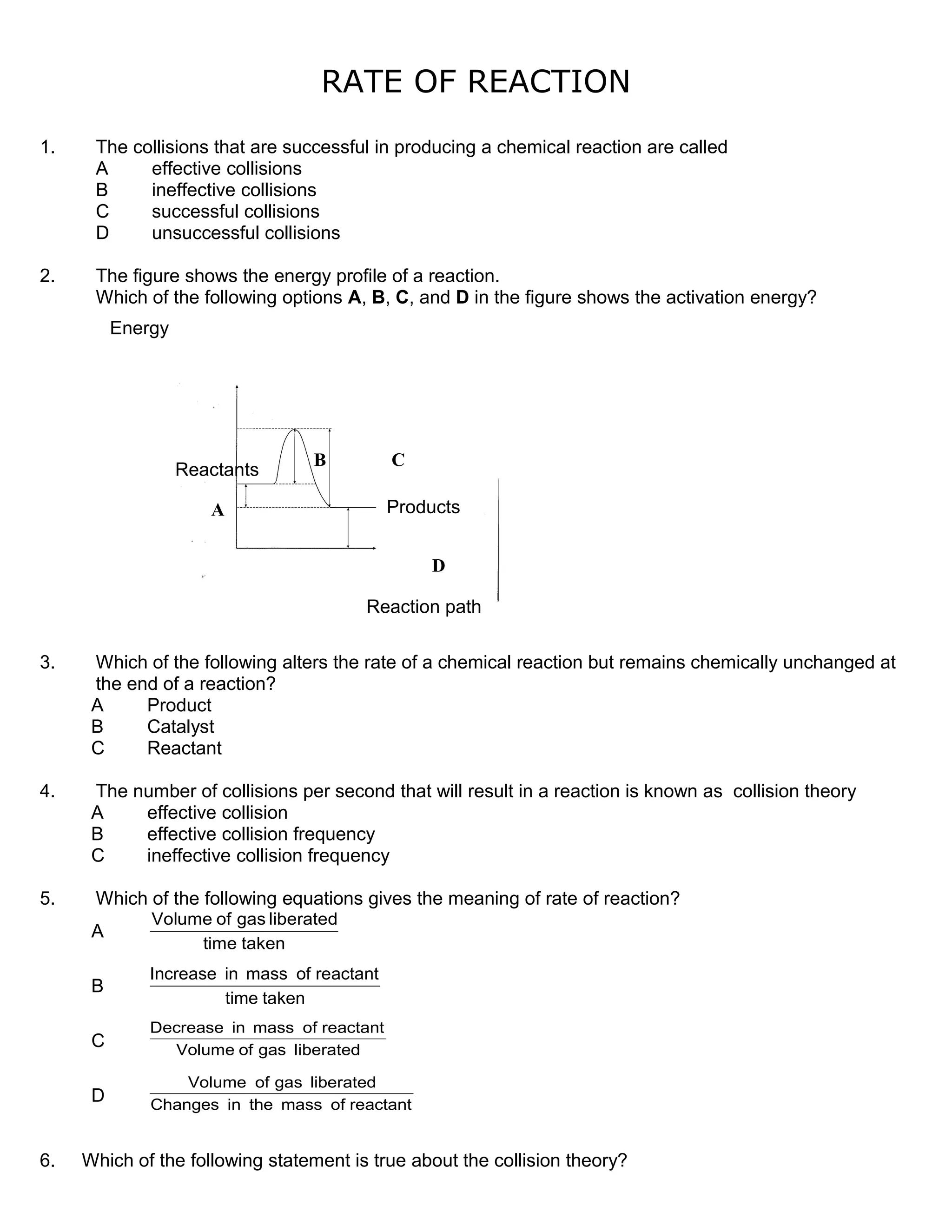
2. Collision theory states that molecules must collide with sufficient energy, called the activation energy, for a reaction to take place. Increasing effective collisions through factors like concentration and temperature increases the rate.
3. A catalyst facilitates the reaction by providing an alternative reaction pathway with lower activation energy, allowing more particles to react per unit time and increasing the rate.