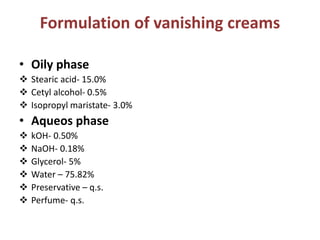
This document defines and describes vanishing creams. It states that vanishing creams are oil-in-water emulsions that leave an almost invisible layer on the skin after application. They contain a high percentage of water and stearic acid, which evaporates leaving a thin film. Stearic acid is the major ingredient and provides a non-greasy feel. Other common ingredients include glycerin, preservatives, and perfumes. The document provides a sample formulation and instructions for producing a basic vanishing cream.