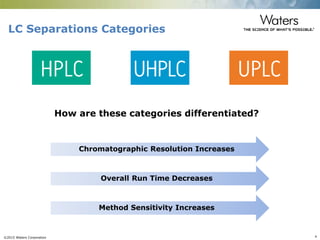
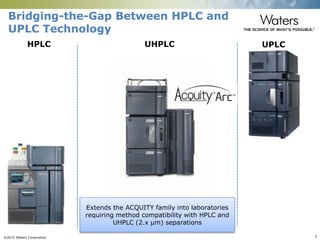
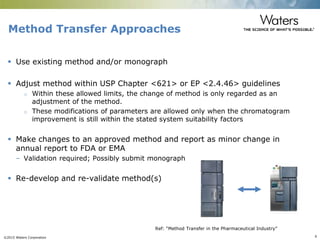
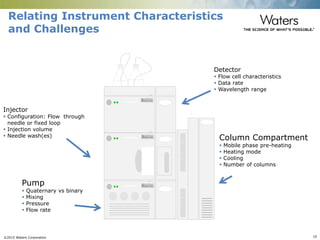
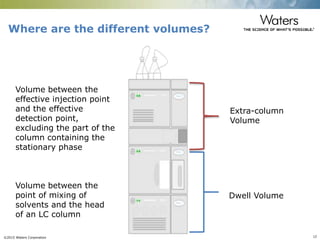
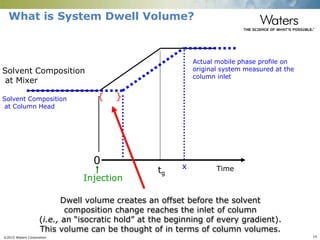
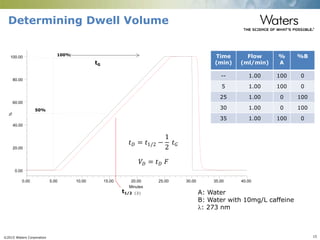
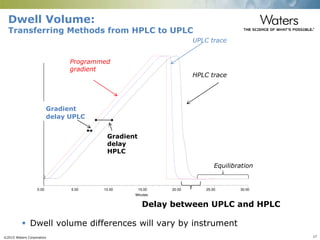
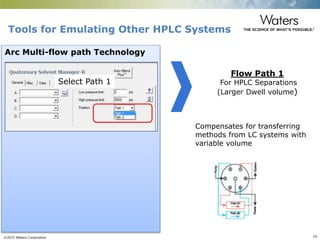
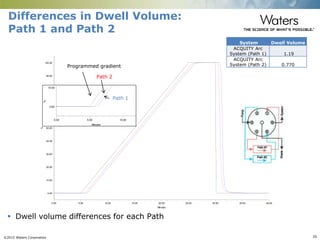
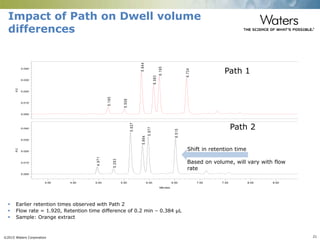
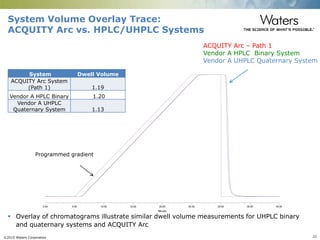
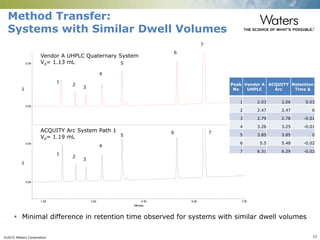
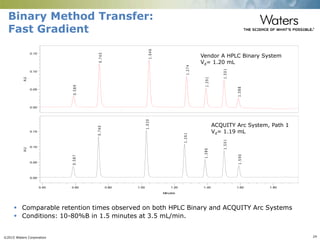
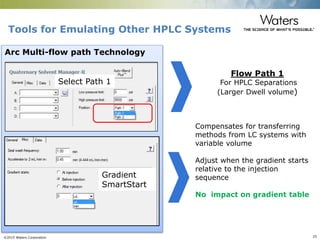
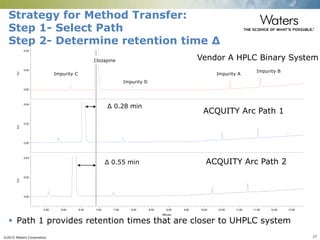
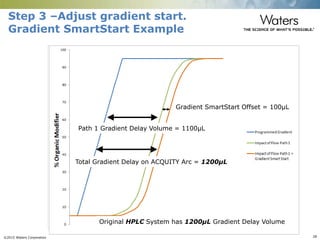
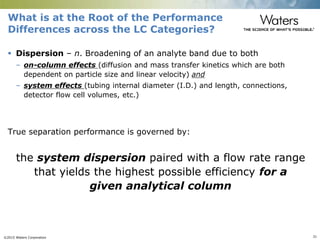
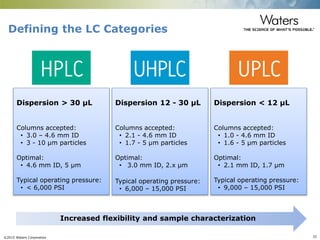
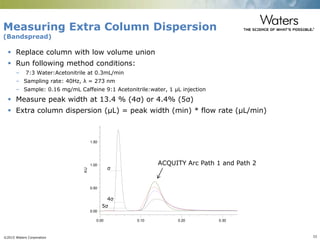
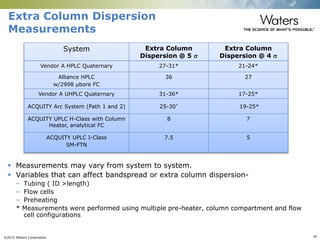
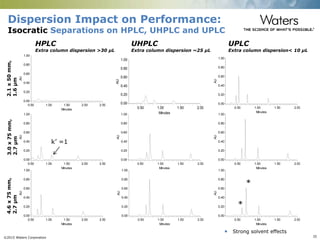
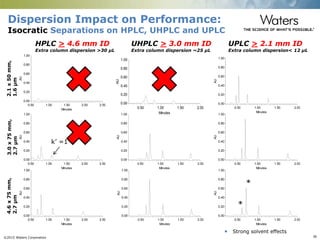
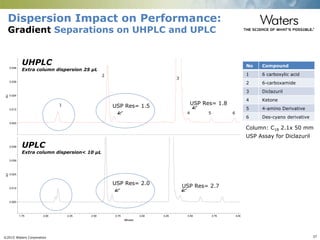
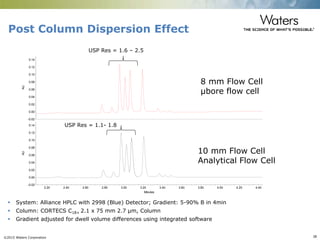
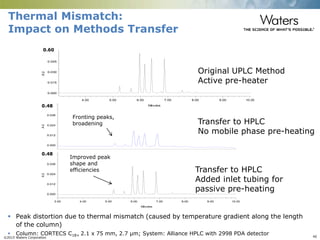
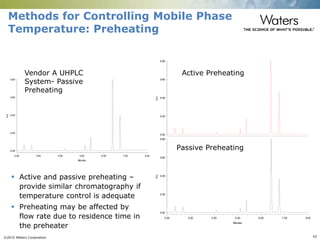
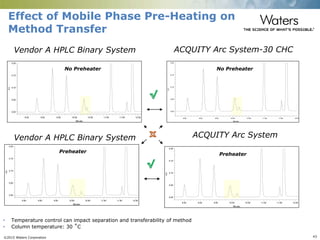
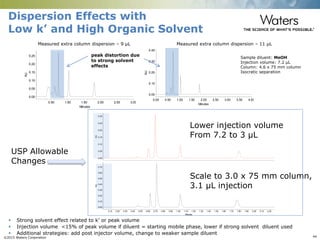
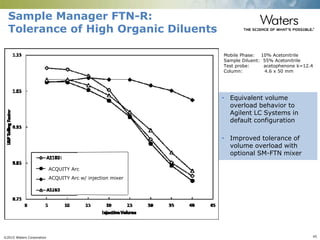
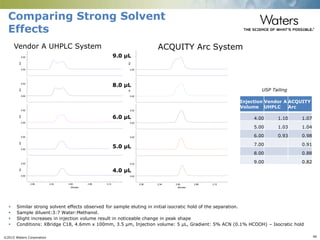
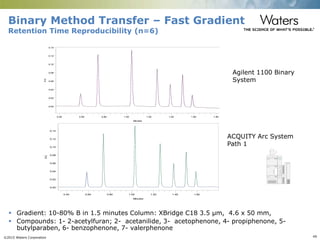
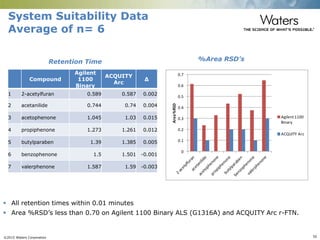
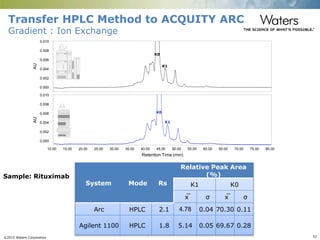
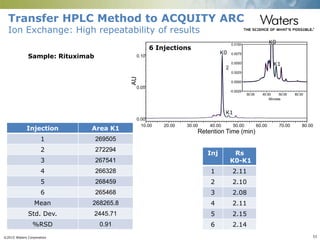
The document discusses the challenges and strategies for method transfer to modern liquid chromatography (LC) systems, specifically focusing on the Acquity Arc system which bridges the gap between HPLC and UPLC technologies. It emphasizes the importance of matching system characteristics like dwell volume, mixing behavior, and gradient profiles to ensure successful method transfers while maintaining regulatory compliance. The text outlines various strategies, including using hardware emulation tools and making necessary adjustments to optimize retention times during method transfers.