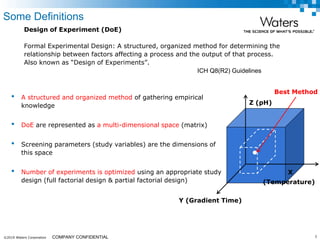
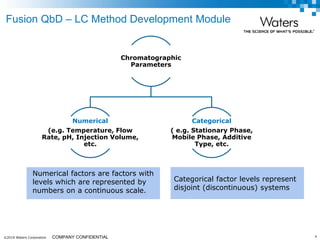
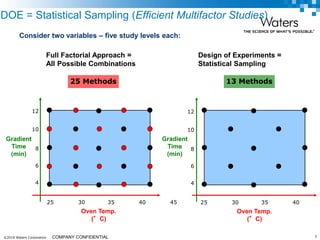
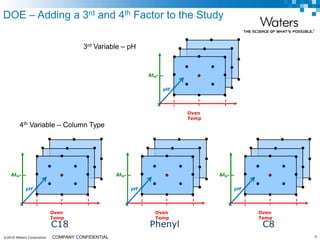
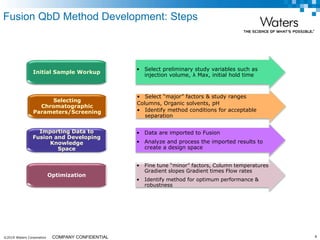
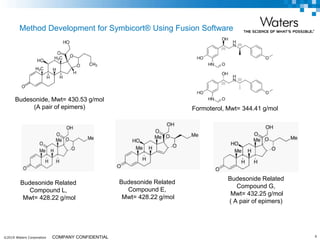
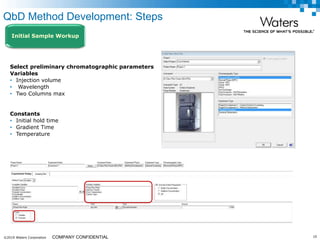
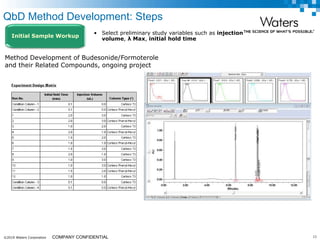
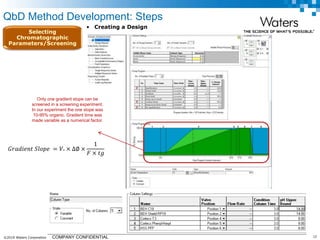
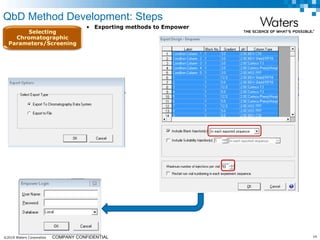
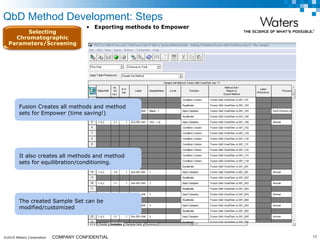
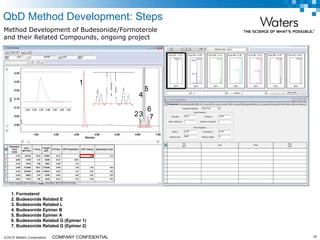
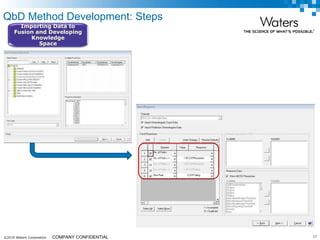
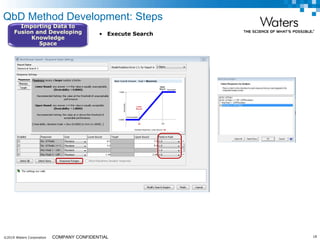
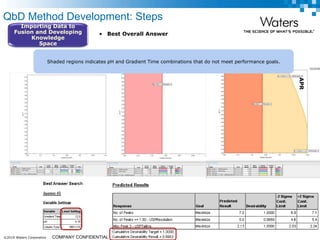
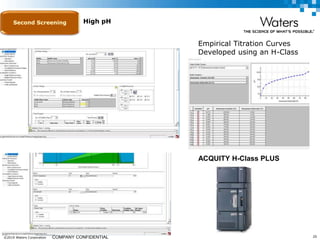
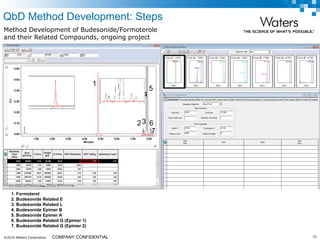
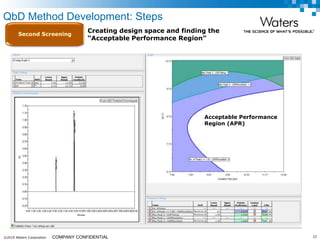
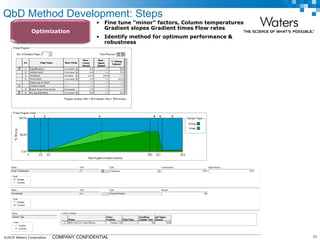
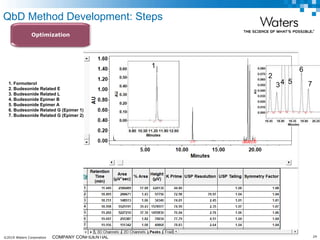
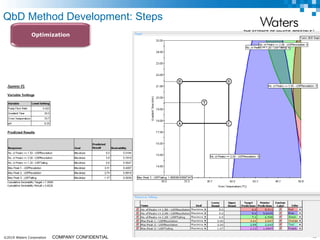
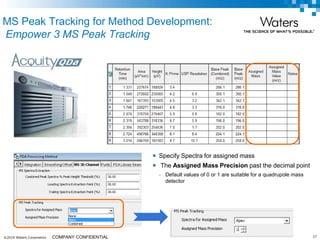
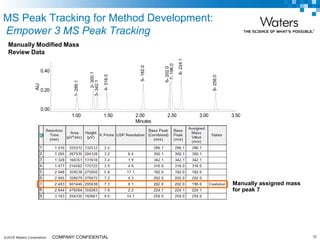
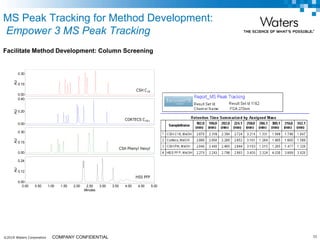
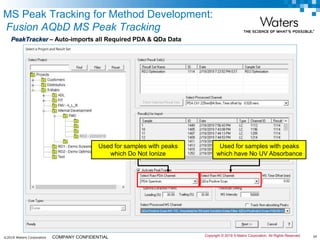
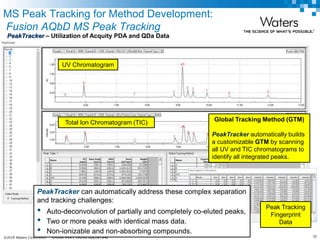
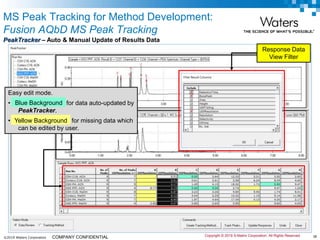
The document discusses the use of Fusion QBD® software by Waters Corporation for analytical quality by design (QbD) in method development, emphasizing a systematic approach that includes defining objectives, using design of experiments, and quality risk management. It details the steps for developing chromatographic methods, including selecting variables, optimizing performance, and employing mass spectrometry for effective peak tracking. The benefits of using Fusion QBD include process automation, time savings, and improved visualization of chromatographic parameters.