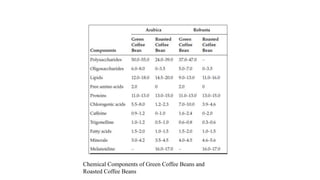
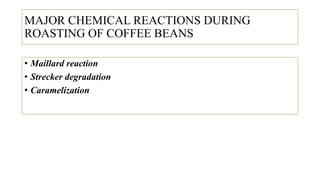
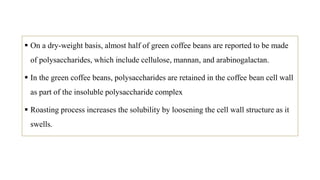
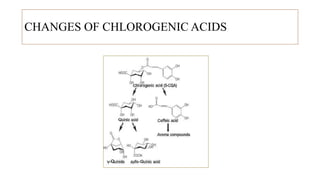
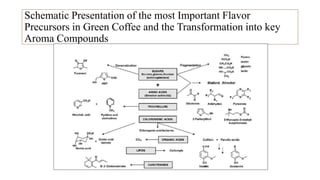
The document discusses the roasting process of coffee beans, emphasizing its critical role in developing flavor and transforming green coffee into desirable roasts. It outlines the major chemical changes during roasting, including the Maillard reaction, caramelization, and the degradation of various compounds, which contribute to aroma and flavor development. Key indicators such as the first and second crack temperatures, colors, and structural changes of the beans are also highlighted as important factors in determining roast quality.