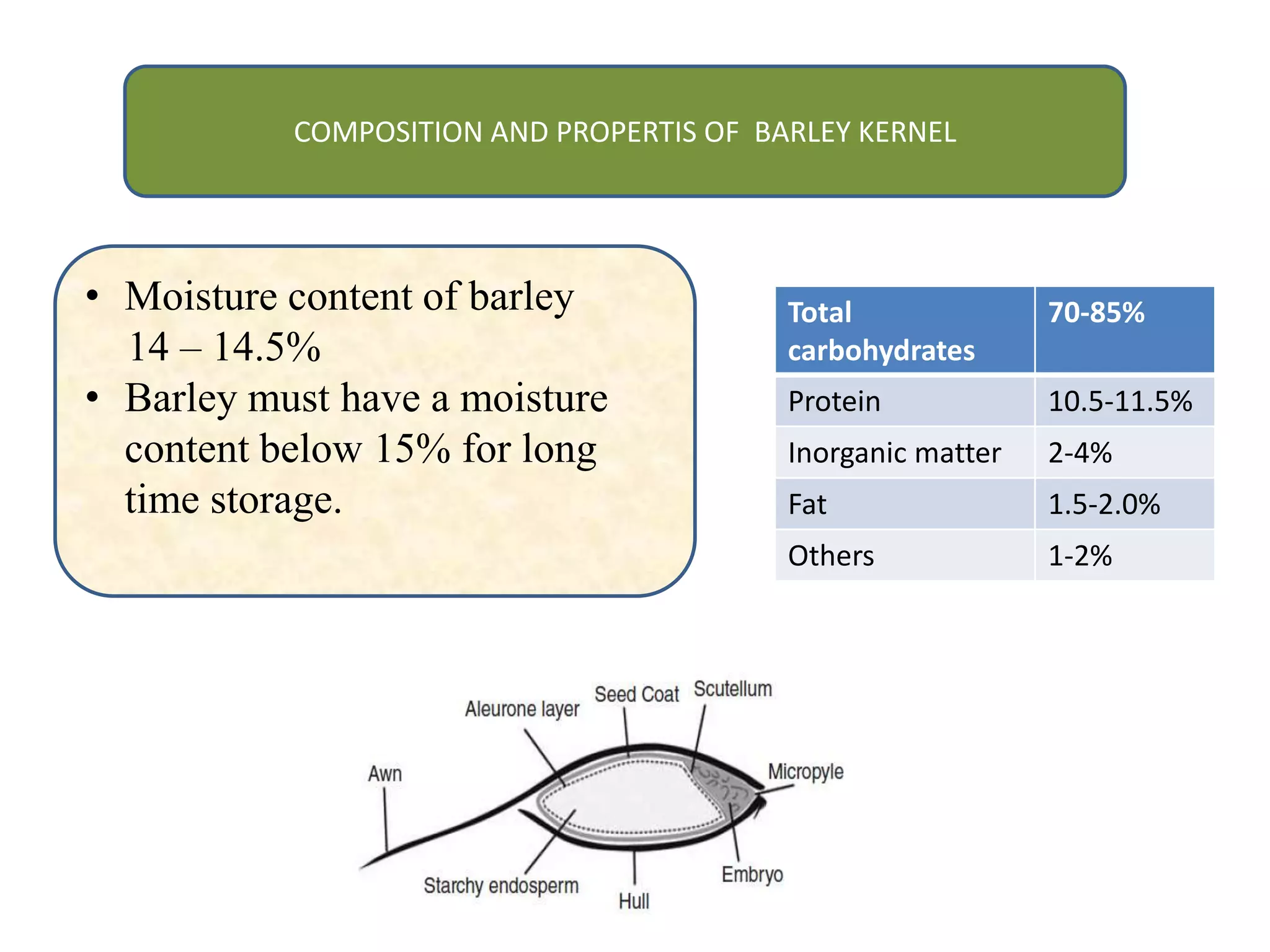
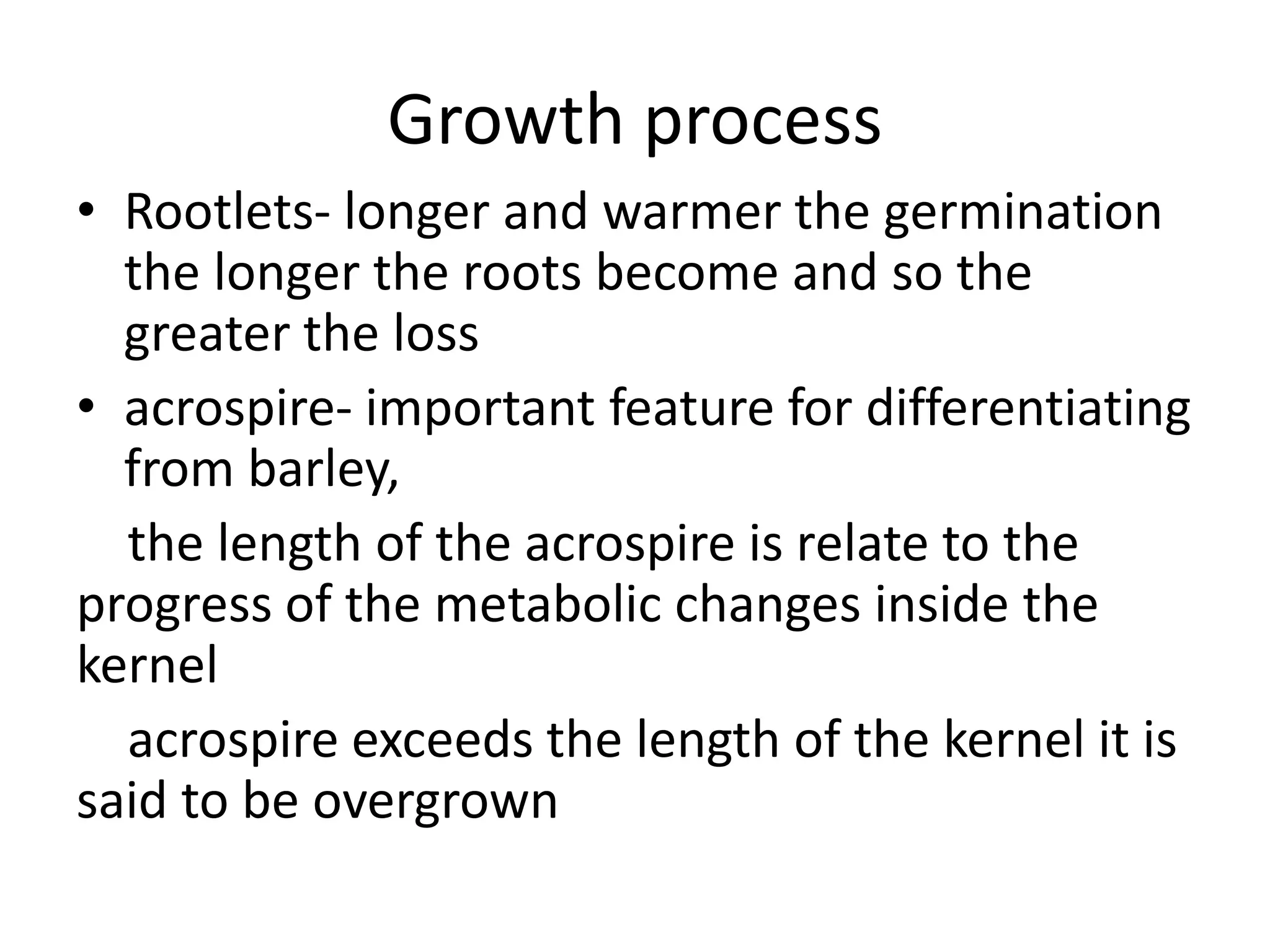
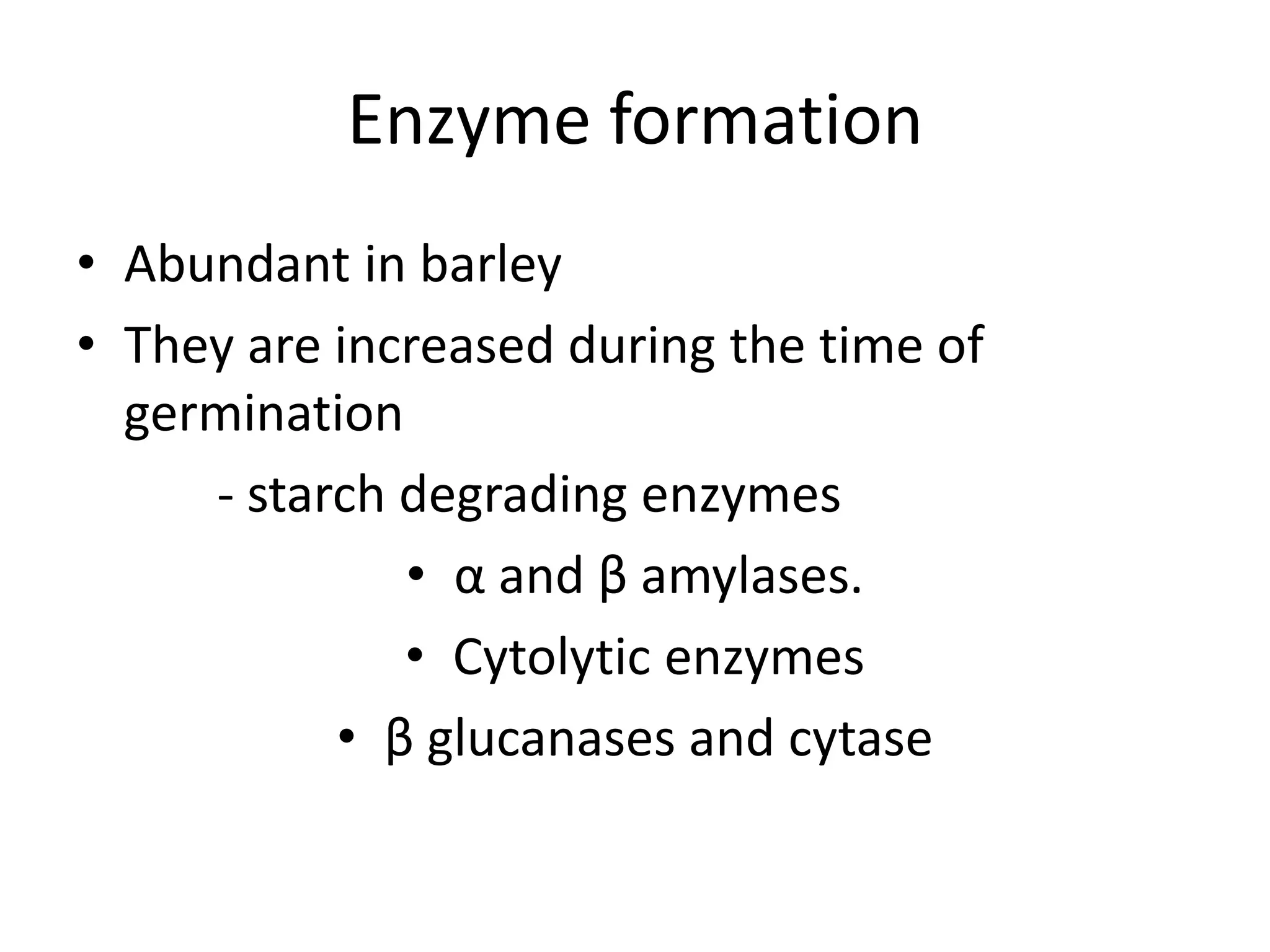
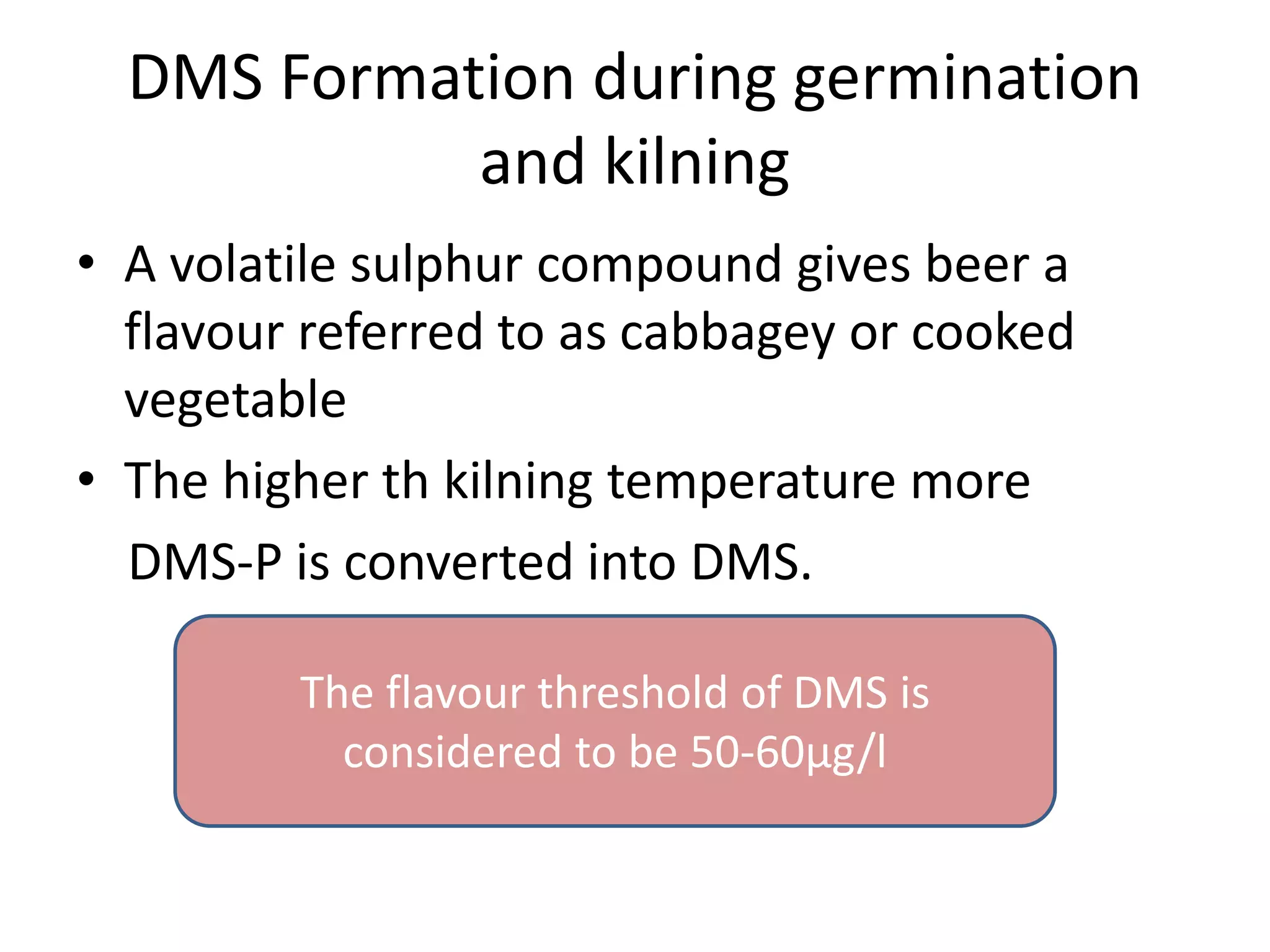
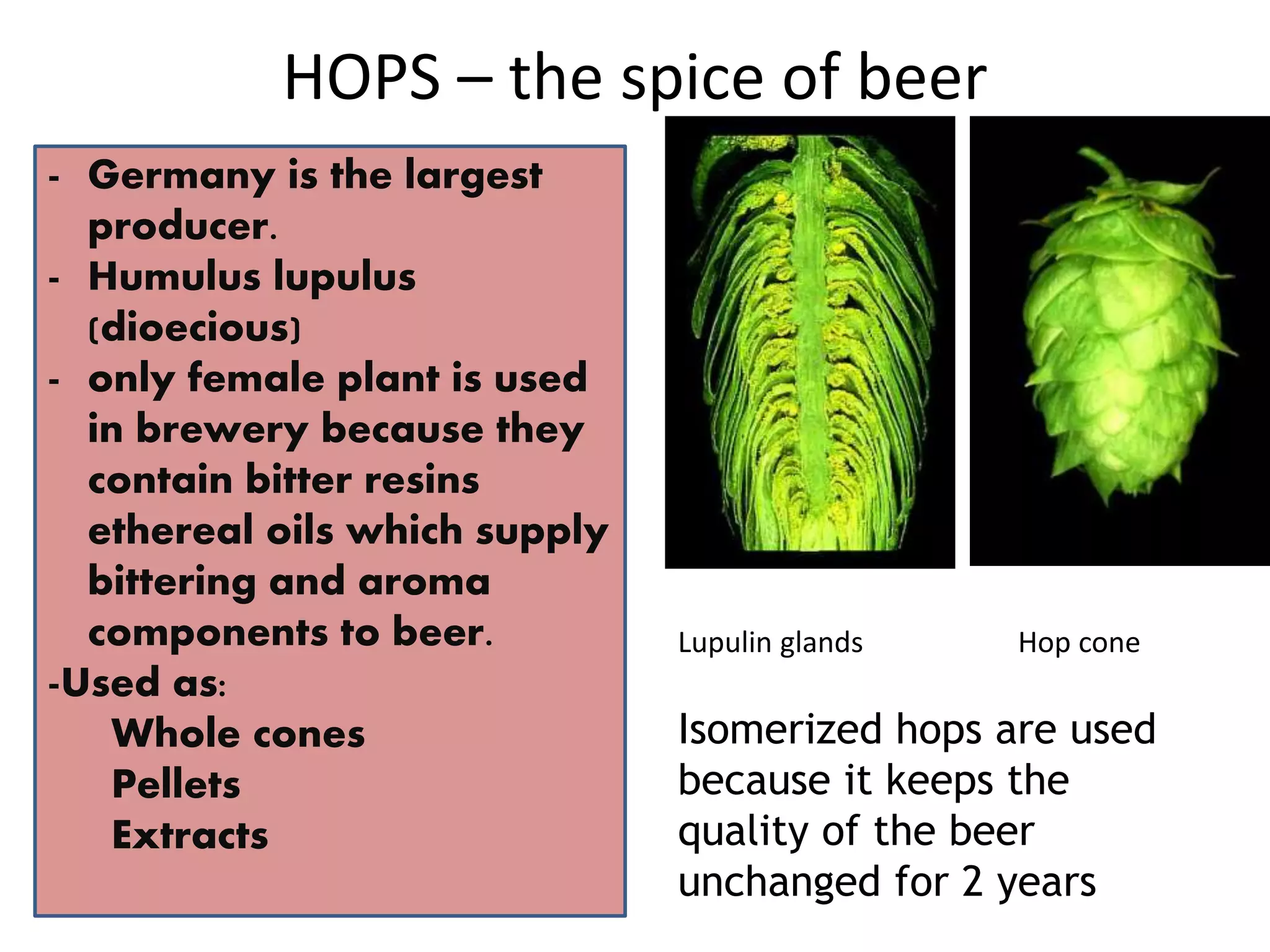
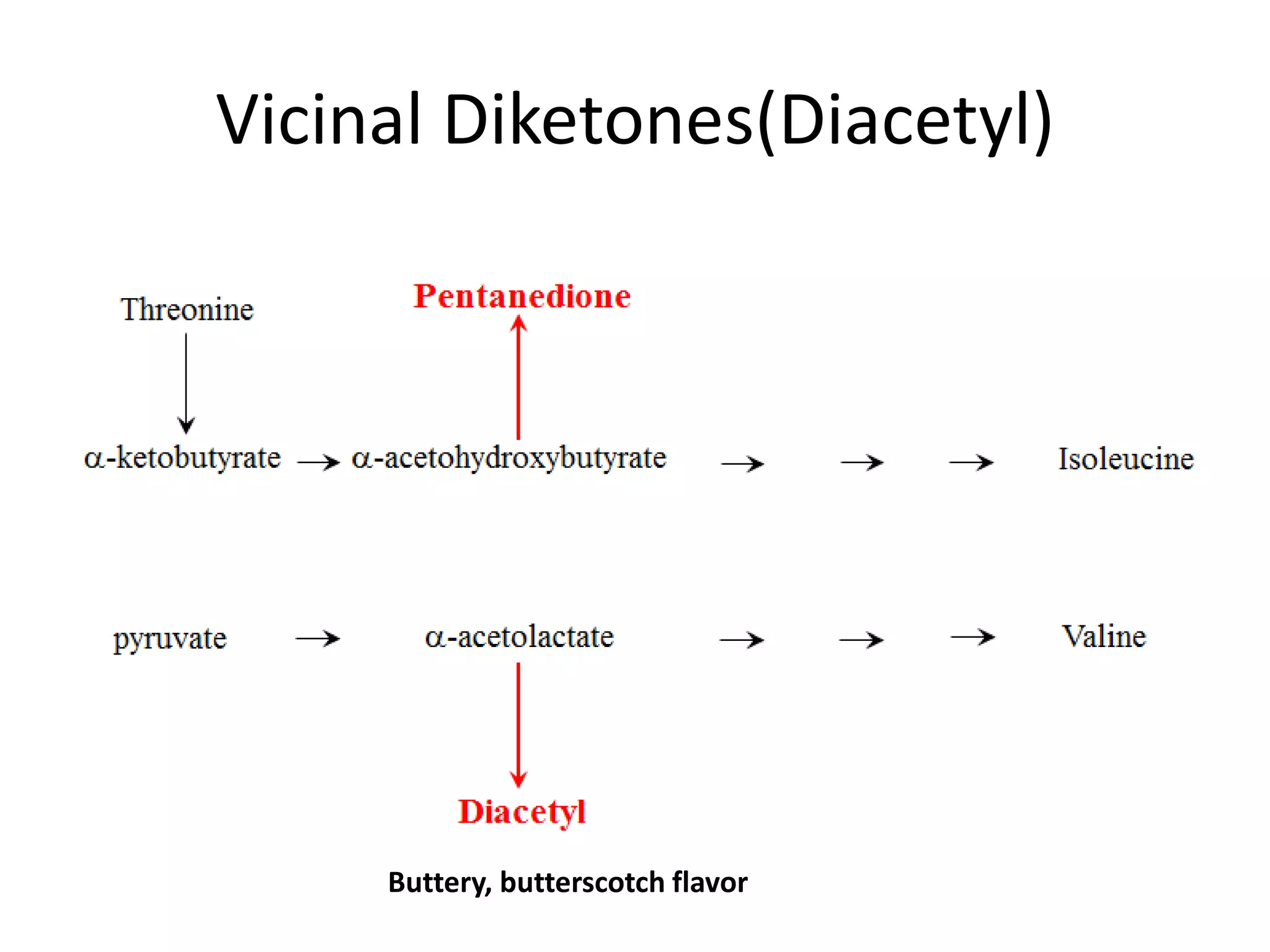
This document discusses the chemistry involved in the brewing of beer. It describes the key ingredients - malted barley, hops, yeast, and water - and the chemical processes they undergo during brewing. Malted barley is mashed to produce fermentable sugars, while hops provide bitterness, aroma, and stability. Yeast ferments the sugars to produce alcohol and carbonation. The document outlines the brewing stages of mashing, lautering, boiling, fermentation and finishing processes and the chemical reactions that occur at each stage to produce beer.