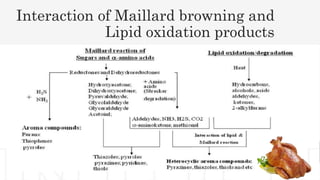
The document discusses the Maillard reaction and how it is used to develop chicken flavor through processing. The Maillard reaction involves the condensation of reducing sugars and amino acids or proteins when heated. This generates flavor compounds responsible for meaty and roasted flavors. Specifically for chicken flavor, the reaction of L-cysteine (a sulfur-containing amino acid) and L-arabinose produces 2-methyl-3-furanthiol, giving the perception of chicken meat. The document outlines the key components, reaction conditions, and processing parameters required to synthetically produce meat-like flavors through Maillard reactions.