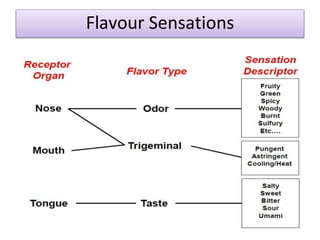
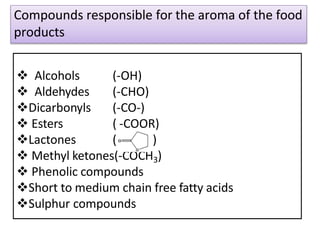
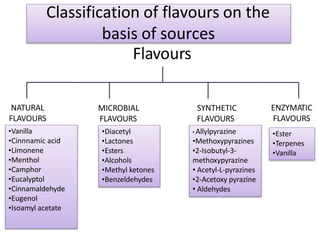
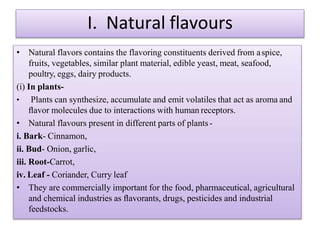
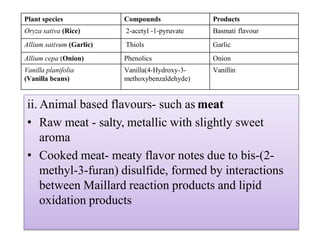
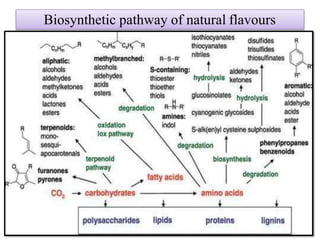
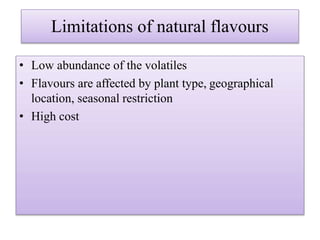
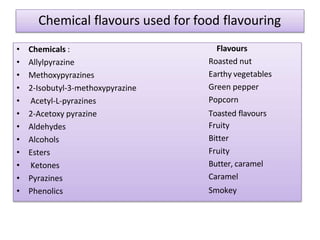
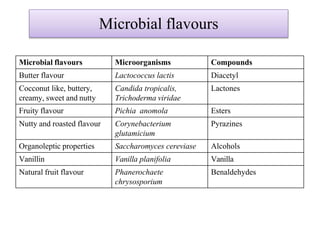
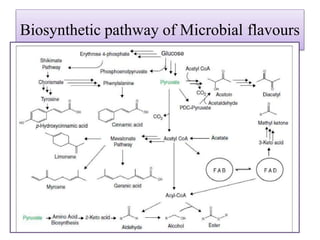
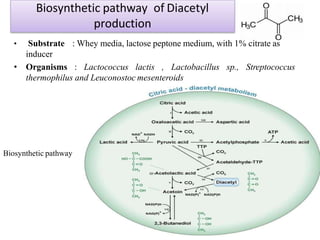
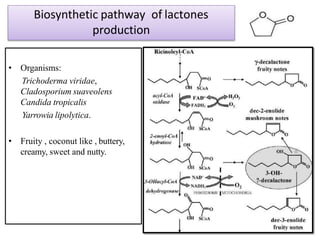
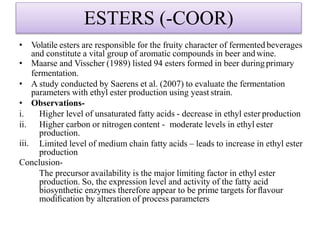
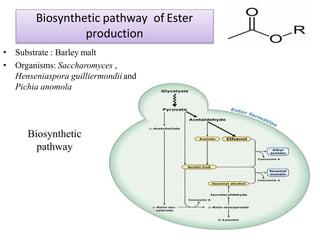
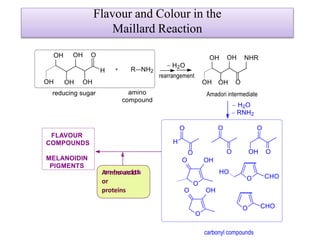
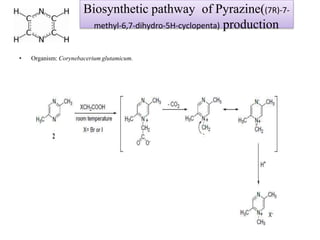
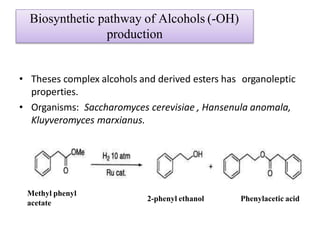
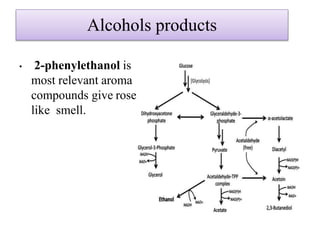
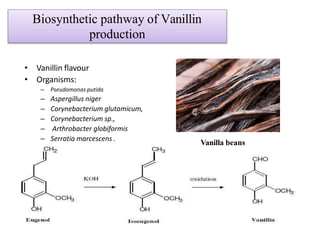
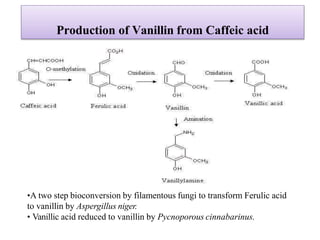
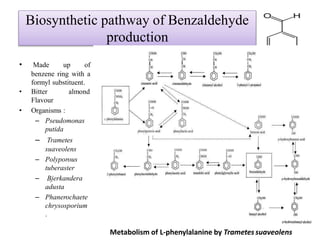
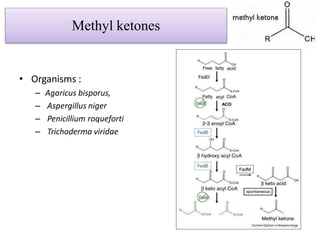
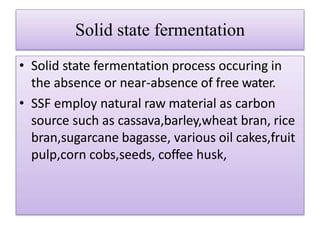
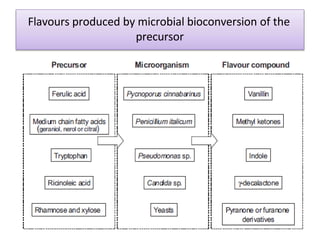
This document discusses microbial metabolites that can be used as flavoring agents in foods. It begins by listing various functional groups found in microbial flavor compounds such as alcohols, aldehydes, esters, and lactones. It then classifies flavors based on their source as natural, microbial, enzymatic, or synthetic. The document goes on to describe the biosynthesis of several important microbial flavors including diacetyl, lactones, esters, pyrazines, alcohols, vanillin, benzaldehyde, and methyl ketones. It discusses the microorganisms and precursors involved in the production of these compounds as well as their applications as flavors. Solid state fermentation is also introduced as a method for