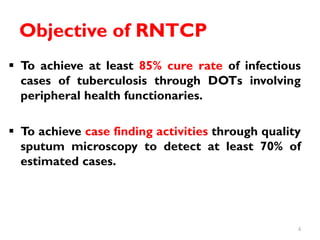
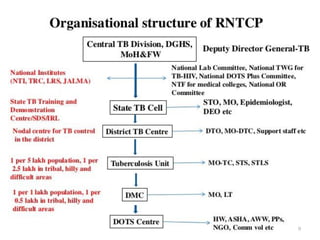
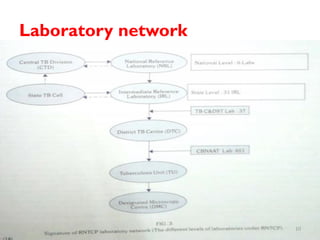
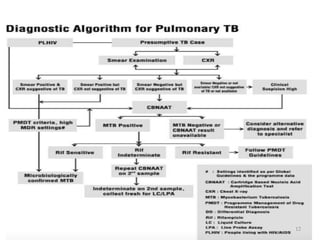
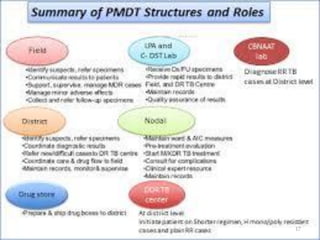
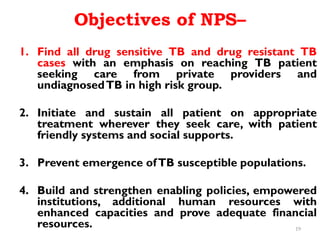
The Revised National Tuberculosis Control Programme (RNTCP) was launched in 1993 in response to low treatment success rates and the threat of multidrug-resistant tuberculosis. It adopted the Directly Observed Treatment, Short-course (DOTS) strategy aiming for an 85% cure rate and a 70% case detection rate through sputum microscopy. The National Strategic Plan (2017-2025) aims for a TB-free India, with specific objectives to reduce TB incidence and mortality and eliminate associated financial burdens by 2025.