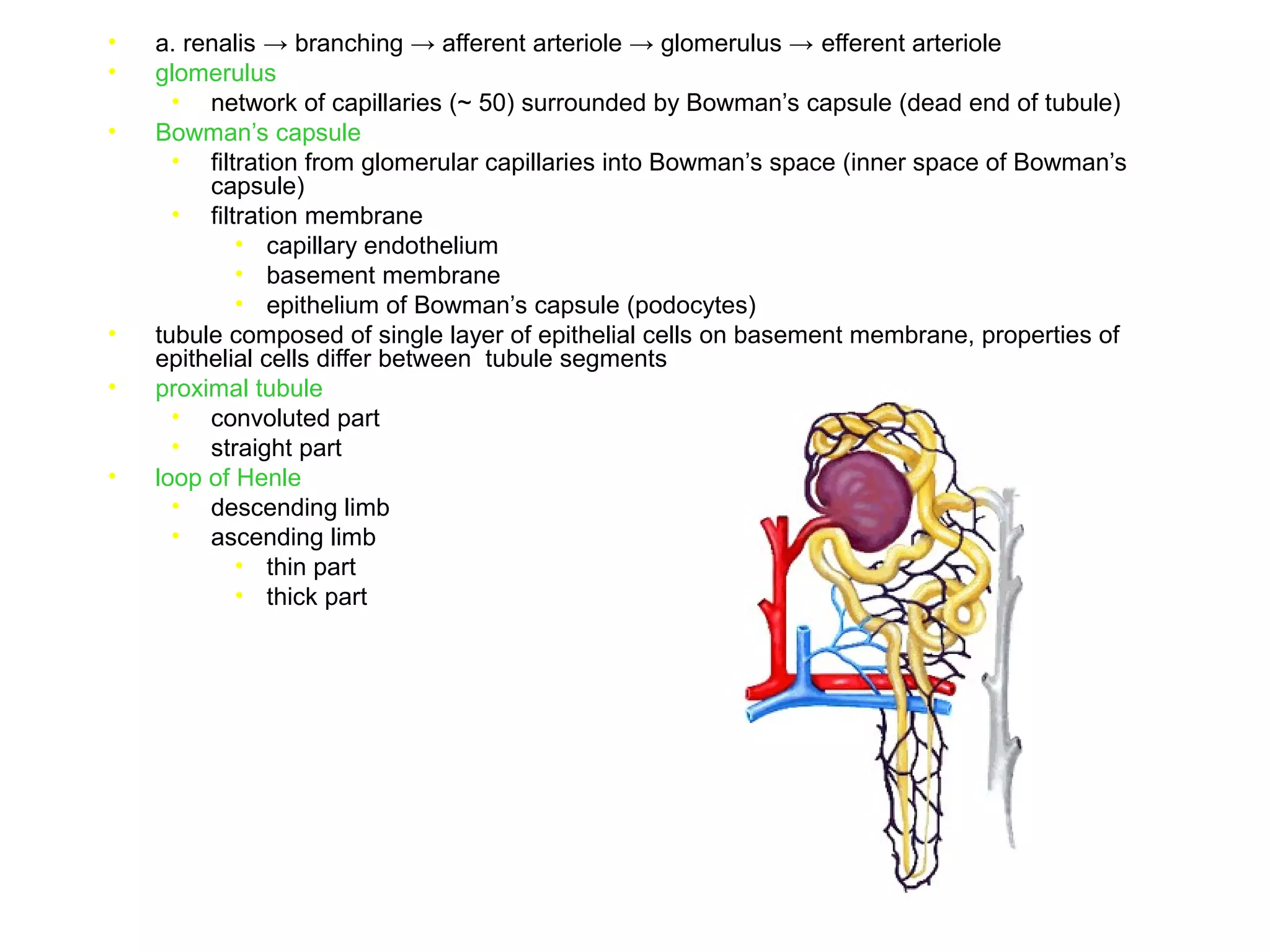
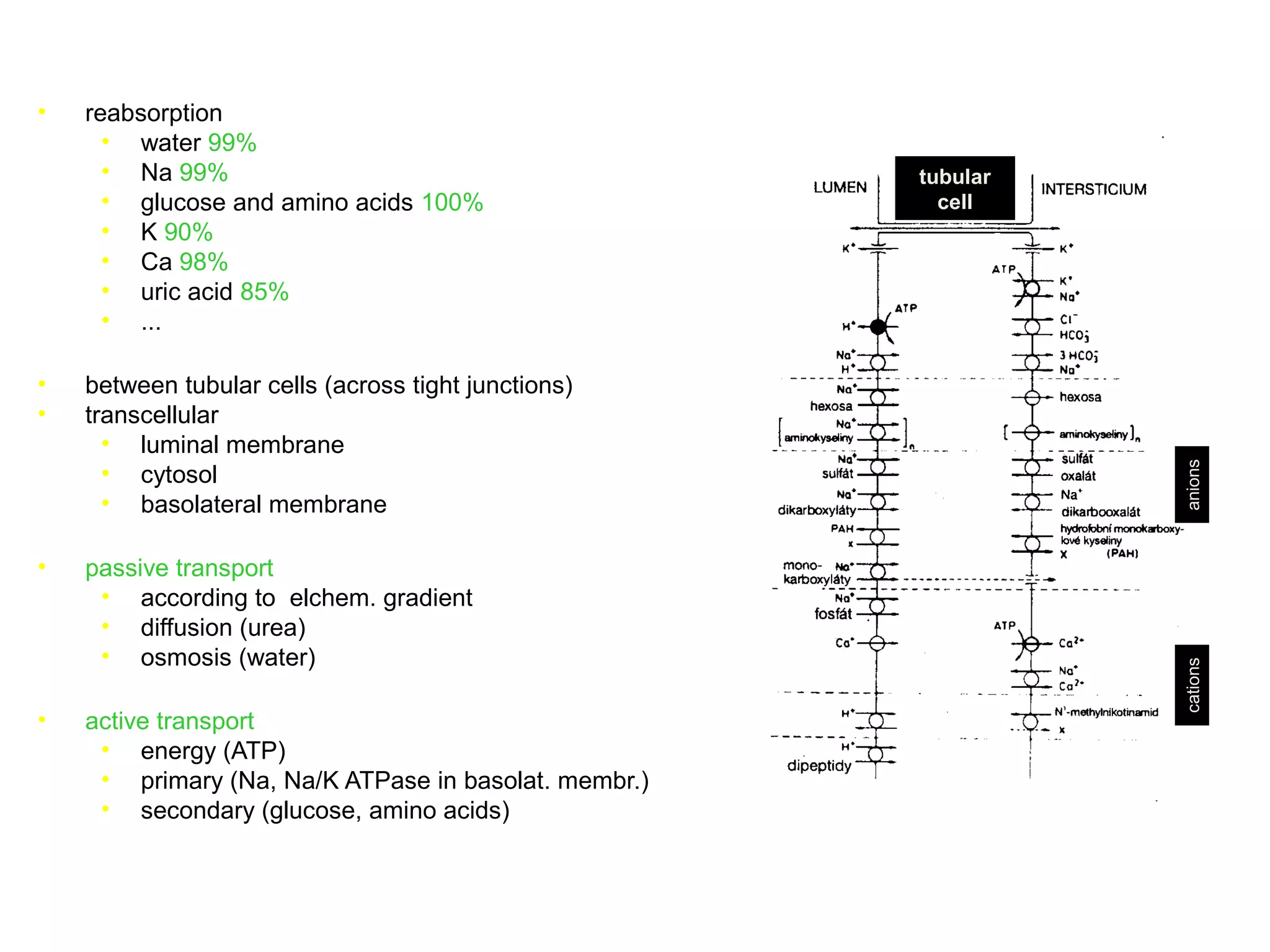
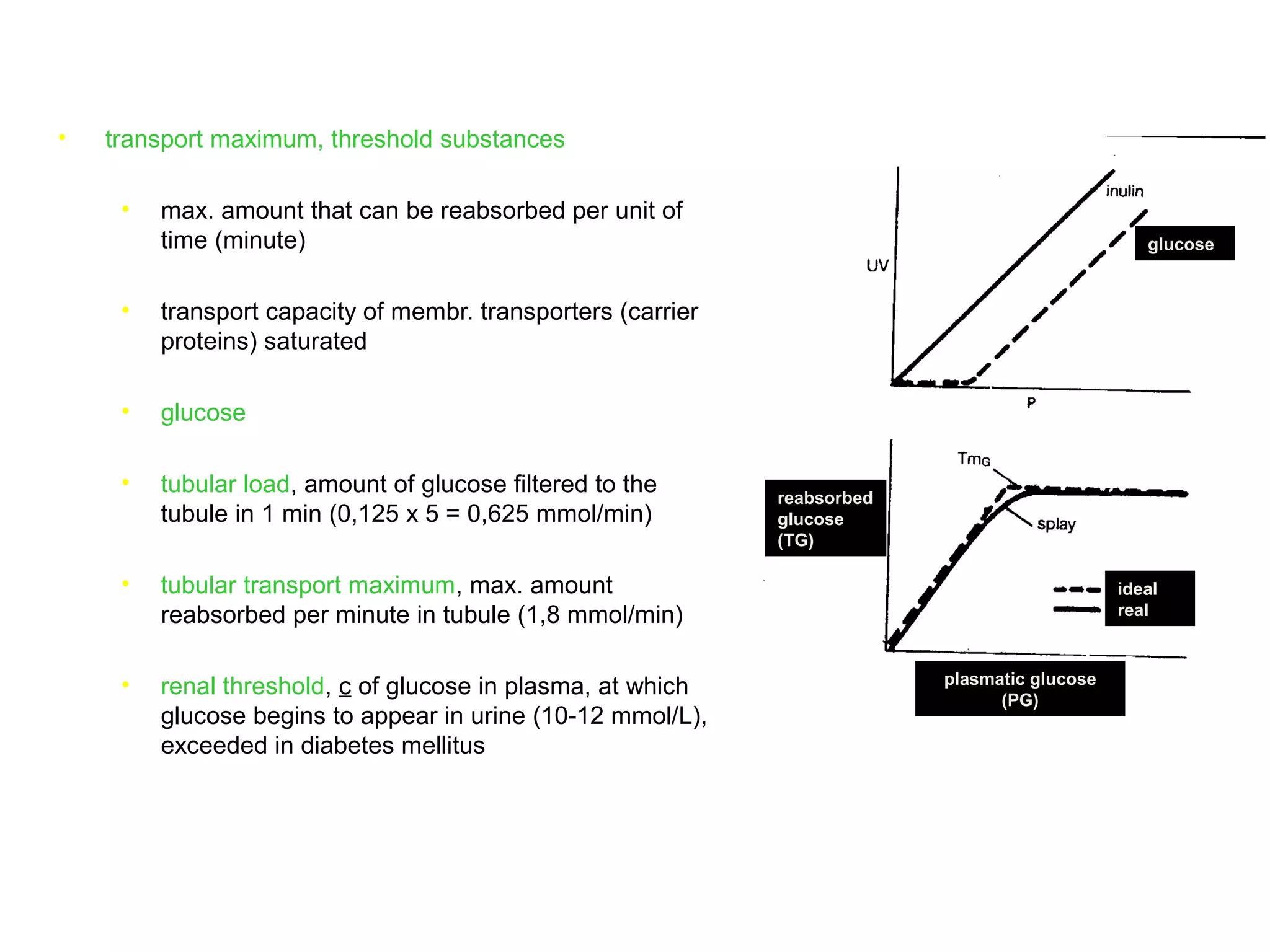
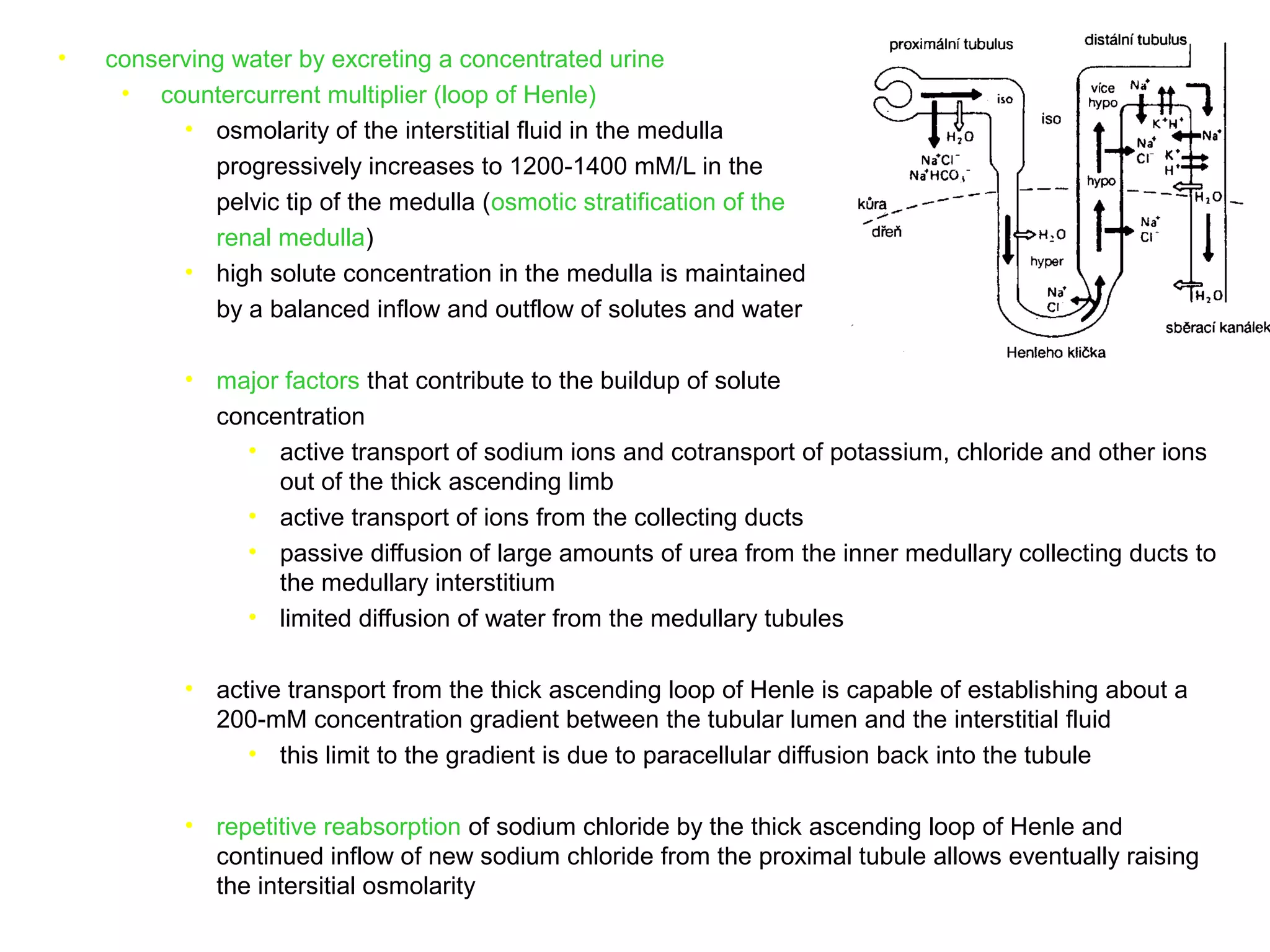
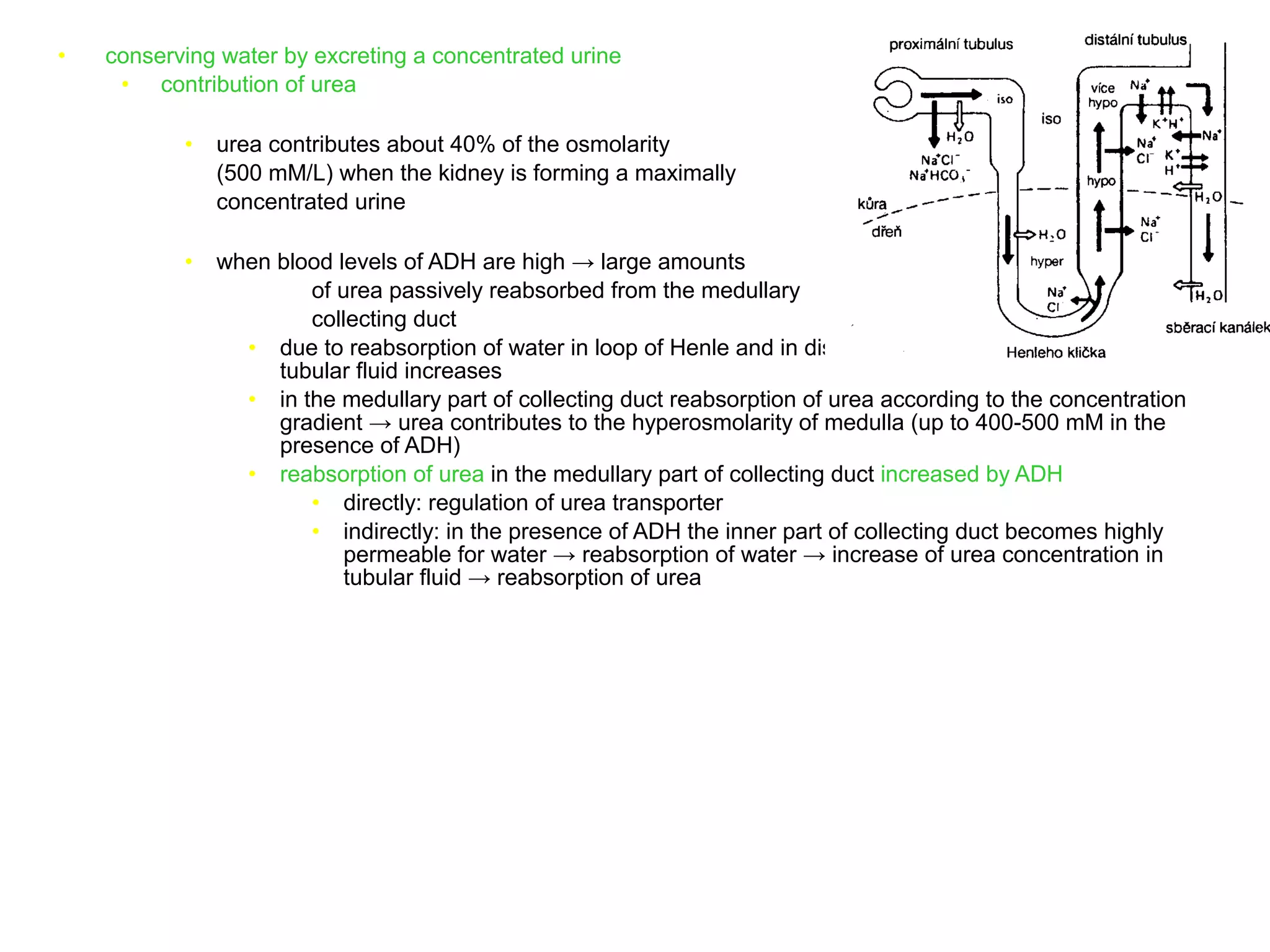
The document discusses the physiology of the kidney. It provides an overview of renal functions such as regulating water, electrolyte and acid-base balance, and excreting waste products. It describes the structure of the kidney including nephrons, glomeruli and tubules. It discusses glomerular filtration, the processes of filtration, reabsorption and secretion, and how clearance is used to measure glomerular filtration rate and renal blood flow.